Wheat Stem Sawfly: Best Management Practices
EB0244
Agronomy Technical Note MT-95
New September 2024
By David K. Weaver, Professor, Department of Land Resources and Environmental Sciences, Montana State University
Susan M. Tallman, CPAg, State Agronomist, USDA Natural Resources Conservation Service, Montana
Tyler J. Lane, Montana State University Extension, Chouteau County
Overview
The wheat stem sawfly (Cephus cinctus Norton) is the dominant pest of dryland wheat production in Montana and across much of the Northern Great Plains (Figure 1). Based on empirical models, it is estimated the average farm can lose 13.5 percent of the winter wheat yield and 11.5 percent of the spring wheat yield in areas with large, damaging populations of the pest, making successful management a top priority for Montana farmers.
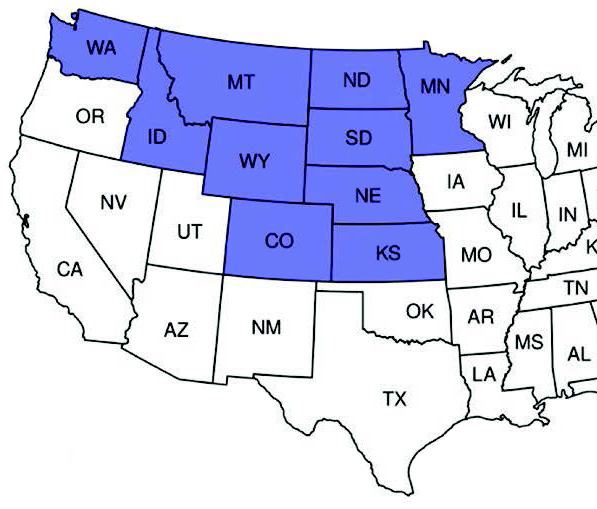
Figure 1. States (purple) with known populations of wheat stem sawfly in the Northern Great Plains. Distribution adapted from and first created by Achhami et al. 2020.
In addition to decreasing grain crop yield and farm income, wheat stem sawfly (WSS) also increases conservation resource concerns in cropland. Frequent stem cutting by WSS larvae below or near the soil surface reduces standing residue height, making the soil more prone to wind erosion. According to the NRCS National Agronomy Manual, “Standing residue is at least three times more effective at controlling wind erosion than flat residue” (USDA-NRCS, 2011). Likewise, shorter stubble reduces the potential for over-winter snow catch and increases soil temperature and evaporative water loss, making cropland less resilient to drought and climate warming. As a result, the challenges from WSS extend beyond decreased crop yield and contribute to a reduction in sustainability and soil health in wheat cropping regions with large pest populations (Figure 2).

Figure 2. Wheat stem sawfly (bottom left) can reduce stubble height and structure causing reduced snow retention and increased erosion. Photo and graphics: M. Hofland and M. Hager, MSU.
Currently, no insecticides provide successful control of wheat stem sawfly. As a result, cultural methods such as variety selection, crop rotation, and harvest management, are the only successful methods of suppression for this pest. T he purpose of this bulletin is to provide producers, crop advisors, and conservationists with practical and effective best management practices (BMP) for wheat stem sawfly management to promote greater economic and ecological sustainability for Montana farmers. The BMPs provided in this document are based on research conducted over the last three decades, both on-farm and in the laboratory, by Montana State University WSS Laboratory staff, graduate, and undergraduate students. Research funding was provided by multiple sources and organizations, with many suggestions and on-farm participation from wheat and barley producers across Montana.
Wheat Stem Sawfly Lifecycle
WSS is a native pest to North America that inhabited the prairie before agricultural settlement (Figure 3). Locally adapted WSS populations gradually adapted from using native plants to the cultivated crops of spring wheat (1900s), winter wheat and triticale (1980s), and barley (2010s). WSS continues to use native plants and as a result, there is always a background population of WSS on the local landscape. Successful management of WSS requires an awareness of the constant local population with the goal of keeping the populations in cultivated crops as small as possible. Complete eradication of the pest is not a realistic management goal.


Figure 3. Adult female (top) and fully grown larva (bottom) of wheat stem sawfly. Photos: RKD Peterson, MSU.
The wheat stem sawfly has only one generation per year. Adults normally emerge between mid-May and early July and live five to eight days. During this time, the females lay 30-50 eggs into the interior pith, or lumen, of wheat stems. Adults typically do not have to travel long distances to reach a suitable host stem and have a limited flight range of a few miles from their emergence location. Purposeful longer f lights are rarely necessary. The period of adult emergence and flight lasts 3-6 weeks.
The egg stage lasts 5-7 days, followed by a larval stage of about one month. The larvae feed up and down the interior of the stem, descending toward the base of the plant, breaching the stem nodes, and almost completely cutting through the stem horizontally (“girdling”). This girdling point can be from about 0.75 inches below the soil surface to about 1.5 inches above the soil surface. Girdling causes the stem to sever and lodge on the soil surface, causing crop yield loss and requiring special harvesting techniques to pick up the lodged grain heads (Figure 4). These lodged grain heads have lower yield than non-lodged heads due to the decreased water and nutrients from the damaged stem.

Figure 4. Wheat stem lodging (displacement of stem shoot from the upright position) with approximately 80 percent stem cutting by larval WSS. Photo: L. Ermatinger and D. Weaver, MSU.
Pest activity that eventually leads to stem girdling is difficult to detect during the vegetative green phase of crop development, as the plant looks normal and pest activity is hidden within the stem interior. The first visual indication of pest damage from grain lodging occurs as the plant dries down, just before harvest, when it is too late to make any management changes for the given crop year. Likewise, it is very difficult to visually estimate WSS crop yield loss, as the human eye cannot easily detect a difference between 30 to 60 percent damage in a robust wheat stand. Both factors make field monitoring and annual adaptation of harvest management an insufficient strategy for limiting WSS-caused losses. Rather, successful management of WSS focuses on prevention and the continuous use of best management practices with cultural methods.
After girdling the stem, the larvae create a plug of frass, or excreted, digested plant material, inside the stem and below the girdling point (Figure 5D). The larvae then overwinter inside the stem below the plug, in sealed chambers (hibernaculae) typically 1-1.5 inches below the soil surface. After winter, metamorphosis begins with spring warming and eventually the pupa transforms into an adult, chews through the plugged tip of the cut stem, and emerges to begin flight as part of the subsequent year’s generation. (See Montana State University MontGuide MT201107AG Wheat Stem Sawfly Biology for more information on the wheat stem sawfly lifecycle.)
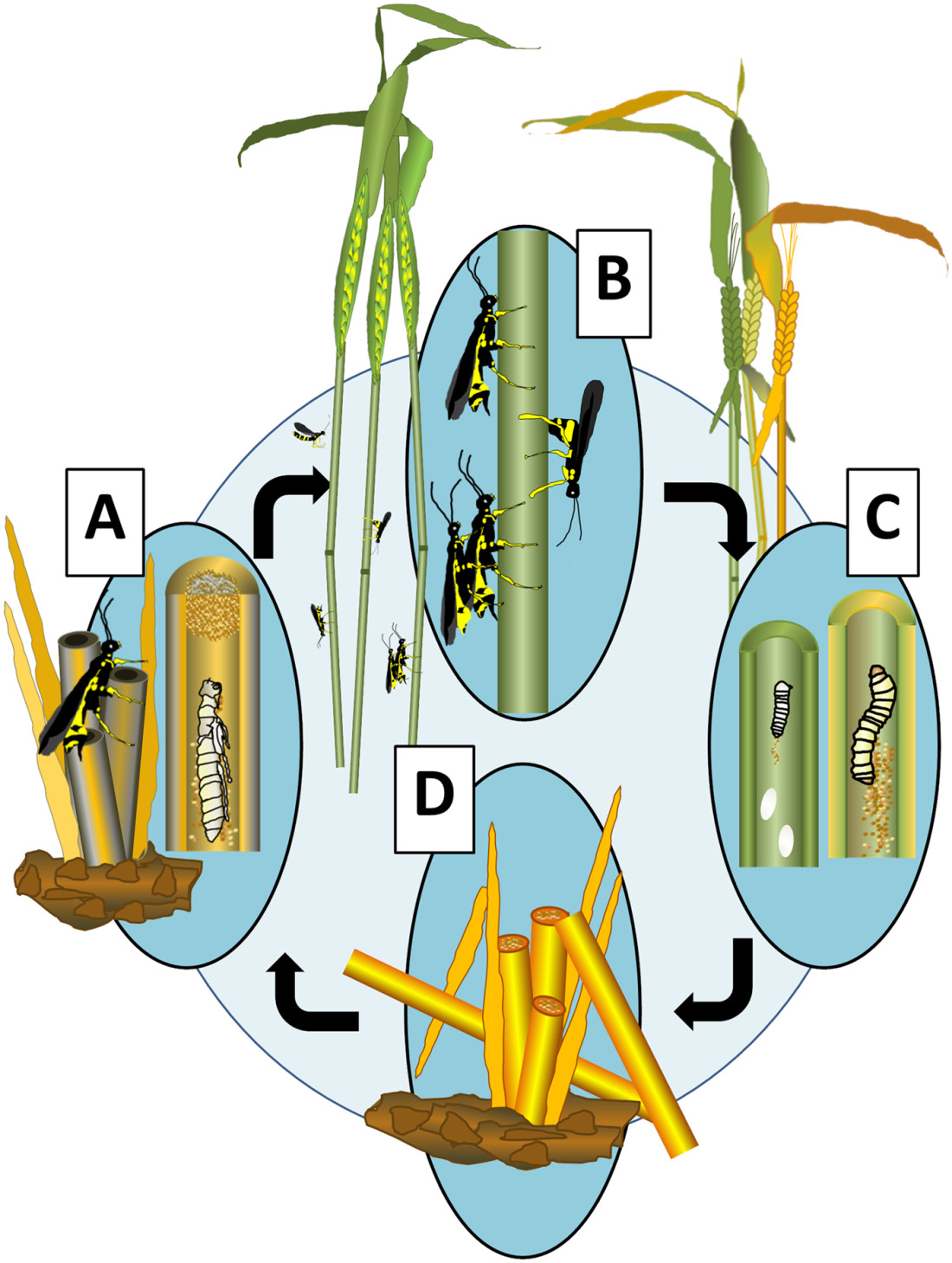
Figure 5. A) Overwintering in wheat stem with metamorphosis in late spring. B) Adults mate immediately after emergence. C) Eggs are deposited in the wheat stem, and larvae feed on the stem interior. D) Surviving larvae migrate within the wheat stem to base at senescence and ripening. Life cycle adapted from Robertson et al. (2018) with image created by M. Hofland and N. Irish, MSU.
Parasitoid Wasp Lifecycle
Insect natural enemies of WSS are also native to North America and are present across the Northern Great Plains landscape (Figure 6). Two species (Bracon cephi and Bracon lissogaster) are parasitic wasps that have two generations per year (compared with only one generation per year of WSS). The adult females paralyze the larvae of WSS, and their offspring consume the immobile larvae. WSS larvae are their only known host. A female wasp deposits one or more eggs near the paralyzed WSS larvae, and when these hatch, the grub-like parasitoid larva feeds on the WSS larva, eventually killing it (killing the host makes these parasitoids rather than parasites).


Figure 6. Adult female parasitic wasps, (Bracon cephi, left) and (B. lissogaster, right). Photos: RKD Peterson, MSU.
In early summer, the adults of these bivoltine parasitoids emerge and live for 30-45 days. During this time, the adults lay eggs into stems infested with WSS larvae. After about one week, parasitoid eggs develop into larvae. These larvae feed on the WSS larvae for a week to 10 days, after which they spin a cocoon for metamorphosis. Inside this cocoon, development into adults takes about one week. The new adults exit the cocoons within the stem and chew a small hole in the side of the stem to emerge from the plant. This generation of parasitoids will mate before harvest and then find a second group of WSS larvae to parasitize and kill. However, these remain in the larval stage within overwintering cocoons inside the wheat stems for 9-10 months, surviving both harvest and winter and emerging as adults early the following summer. Most of these overwintering larval cocoons of the parasitoids are in the lower third of the stem, but they can be found at various heights along the stem length, wherever they initially paralyze and kill the WSS larval host. As a result, conserving standing stubble and maximizing stubble height is very important for WSS suppression, as it protects crucial overwintering habitat for the beneficial parasitoids.
Best Management Practices
Agricultural producers can suppress WSS by using best management practices such as resistant variety selection and proper residue management. Often, the best management practices for WSS control are the same as those for increasing soil health; maintaining adequate soil cover and residue, minimizing soil disturbance, increasing biological diversity, and keeping a living root in the soil (Figure 7).
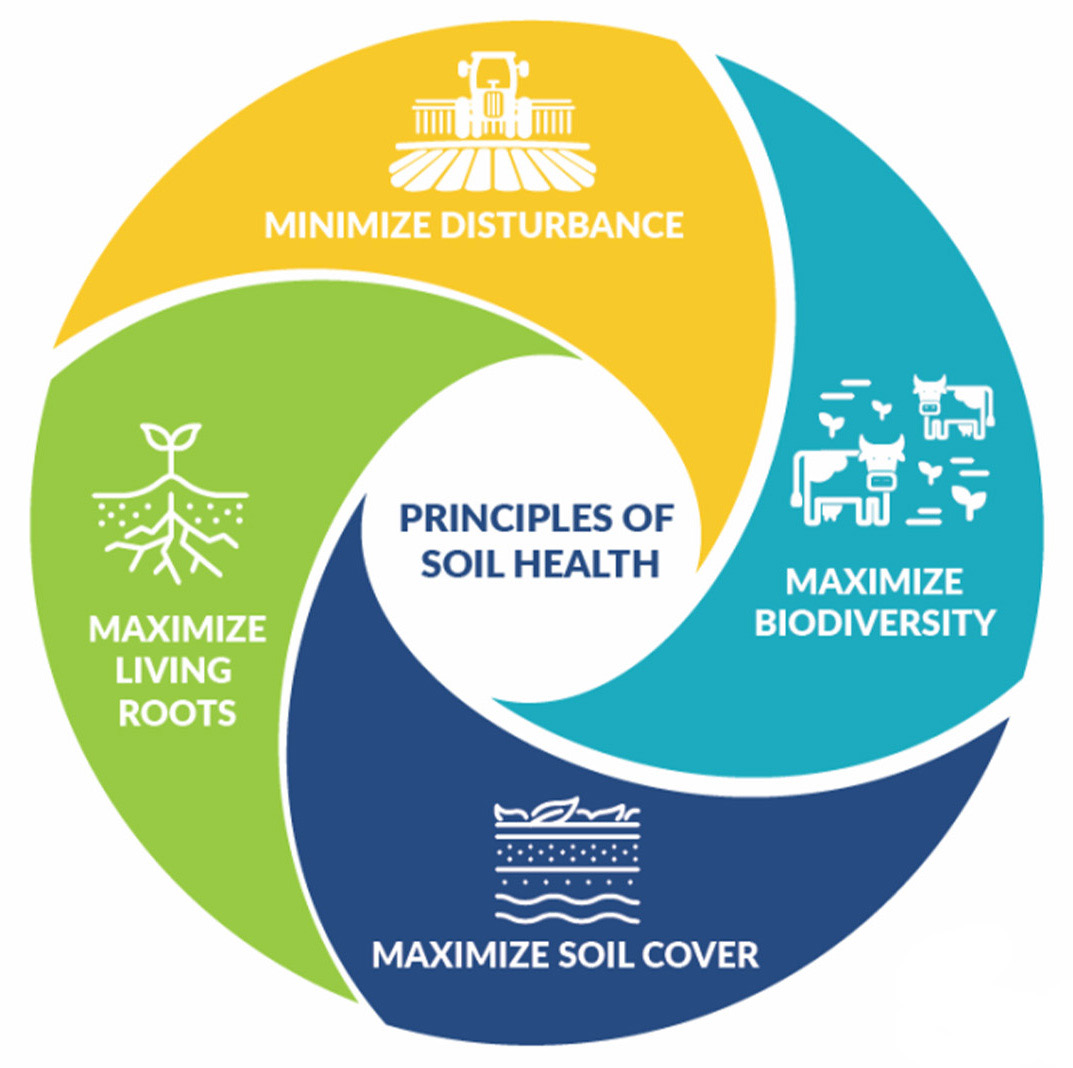
Figure 7. NRCS soil health principles are also beneficial for control of wheat stem sawfly.
Best management practices for WSS control include:
- Plant solid-stem wheat varieties. This is the most foundational and critical management practice for WSS management.
Solid-stem varieties kill about 40 percent of WSS larvae as they feed in the pith
of the stem in the spring (Figures 8 and 9). Producers often do not choose solid-stem varieties, as the yields of these varieties
can be slightly lower (3-5 percent) than hollow-stem varieties. However, infested
hollow-stem varieties may yield 1525 percent less than uninfested hollow-stem varieties,
greatly outweighing the slight yield decrease of solidstem varieties. Note that solid-stem
varieties should only be grown in fields with significant WSS pressure to minimize
the potential for the pest to develop resistance to the solid-stem trait. Lists of
solid-stem winter and spring wheat varieties can be found at plantsciences. montana.edu/foundationseed/quickfacts/.


Figure 8. Solid-stem wheat variety (top) compared with a hollow-stem wheat variety (Yellowstone, bottom). Solid-stem varieties should be used only in areas with wheat stem sawfly infestations. Photos: USDA-NRCS.
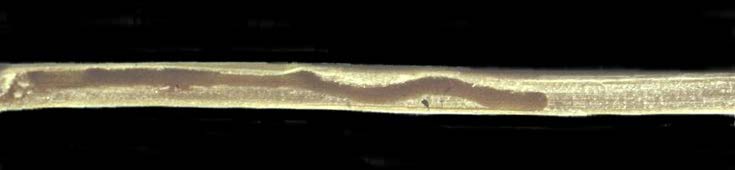
Figure 9. Dead wheat stem sawfly larvae; newly hatched from eggs inside solid stem wheat pith (top, Photo: E. Nichols, MSU) and larval feeding trail ending (i.e., a dead, larger larva) when solid stem wheat pith dries and hardens to become impenetrable (bottom, Photo: W. Morrill, MSU). - Conserve parasitoid wasp residue habitat by leaving wheat, barley, and triticale stubble
as tall as possible. Parasitoid wasp larvae require tall, standing stubble as a habitat to survive through
harvest and the winter. They will not survive inside residue that passes through the
combine chopper or is compressed into straw bales.
Conserve tall, standing residue by cutting wheat, barley, and triticale above at least one-third of the total plant height. For example, if the wheat crop is 27 inches tall, set the combine or swather cutting level at nine inches or greater (Figure 10). Header or swather height should be increased as harvest progresses from the field edge towards the field interior, and farmers can gauge when to do this by visually assessing stem cutting while driving around the field, progressing from the edge to the interior. After several passes, the worst of the typical WSS edge-effect infestation has often already been cut, and there is more to gain from parasitoid conservation via a raised header than the occasional loss of some WSS-cut stems. Likewise, cut stems farther into a field are more likely to be held up by the surrounding stubble than the stems along the field edge where WSS cutting is much worse, making harvest recovery of the stem in the crop interior more likely.
The combination of solid-stem varieties and tall, standing residue is complementarily beneficial. Several years of these practices together should greatly reduce the damaging WSS population size.
Figure 10. A stripper header leaves tall stubble which provides habitat for parasitoids of the wheat stem sawfly. Photo: USDA-NRCS. - Use a no-till management system. No-till systems preserve the standing stubble and maintaining 70 percent residue
cover is needed for parasitoid habitat (Figure 11). Heavy tillage buries residue and kills parasitoids while minimally affecting WSS
as the adults are large enough to dig through the soil after metamorphosis. In general,
single- or double-disc drills are best for WSS management rather than hoe drills,
as they have a greater ability to seed into tall, standing, no-till stubble (Figure 12). Hoe drills can plug when seeding into tall, standing stubble because the residue
is captured on the shank and does not readily dislodge.
However, hoe drills may achieve better seed-to-soil contact in specific situations, including seeding into lodged, horizontal stems from WSS damage and in drought conditions on heavy clay soils. If using a tilled system, use light tillage methods that leave more than 70 percent residue cover on the soil surface to conserve parasitoids and their habitat.
Figure 11. Approximately 70 percent stubble cover on a field. Leave this much or more small grain residue to provide adequate parasitoid habitat. Photo: USDA-NRCS.

Figure 12. A single-disc (left) or double-disc (right) drill is often needed to implement a high-residue, no-till management system, and to successfully seed into tall, standing residue. Photos: USDA-NRCS. - Do not burn residue. Burning of standing residue, either intentionally, or from natural (lightning) or accidental (sparks from equipment on rocky ground) causes, is devastating to parasitoids. All WSS larvae in standing residue are contained in sealed hibernation chambers within the stems below the soil surface. The f leeting heat from a residue fire is neither intense nor long-lasting enough to kill WSS larvae, but it will kill all parasitoid larvae in the standing straw.
- Do not re-crop wheat, barley, or triticale in a field with an existing WSS infestation. Planting wheat after wheat or barley optimizes WSS population growth and should be avoided in areas with damaging levels of WSS.
- Grow oats, a lethal trap crop for WSS. Oats (Avena sativa) have long been known to be a viable trap crop for WSS (Figure 13). Adult female WSS readily lay eggs in oats, and 100 percent of the feeding larvae
eventually die. Consider planting oats at least once in the crop rotation in WSS-infested
areas and leave tall, standing oat stubble to increase snow-holding capacity and reduce
the risk of wind erosion. Time the planting of the oats early enough to provide sufficient
vegetation (stem elongation) during WSS flight and egg-laying activity, typically
a 6-week time window from late May to early July.
Use one of the following methods to include oats in the crop rotation:- Oats grown as a grain cash crop.
- Oat trap crop planted around the perimeter of a wheat, barley, or triticale crop. The oat trap crop should be a minimum width of one seeder pass around the field perimeter, or approximately five percent of the total field acreage. Plant the trap crop within 2-3 days of the wheat or barley crop (preferably before) and use the same crop season type for the trap crop as the attractant crop. For example, plant spring oats with spring wheat and winter oats with winter wheat. In northern latitudes with no winter-hardy oat varieties, spring oats can be planted as a trap crop with winter wheat if the spring oat is planted as early in the spring as possible.
- Cover crop or forage crop with a minimum 15 percent oats as a portion of the total seed mix. Timing of cover crops with oats is crucial for successful WSS suppression. Cover crops with oats planted later in the summer or fall after the 6-week May to July WSS flight window will not be effective. Plant the cover crop at a time when the oat growth stage will match the growth stage of adjacent wheat or barley cash crops.
- Replace fallow years with a non-host crop. Adult WSS emerging in a fallow field exert minimal effort to f ind an adjacent wheat
crop to infest. They simply fly upwind and land at the nearest host crop (wheat, barley,
or triticale). In contrast, adult WSS emerging in a nonhost crop field (pea, lentil,
canola, or safflower) will lose time and energy foraging in the non-host crop before
eventually leaving to find a host crop. Because adult WSS are short-lived (5-8 days),
any lost time and energy f inding a host crop results in fewer eggs being deposited
when they do find it.

Figure 13. Oats (Avena sativa) are a lethal trap crop for wheat stem sawfly. Photo: USDA-NRCS. - Plant a more attractive wheat variety on the outside field border of a less attractive wheat variety. Wheat varieties differ in their attractiveness to adult female WSS by producing different amounts of volatile compounds. Using a more attractive variety on the exterior border will limit WSS damage to the field interior. Informed wheat breeders should have records of which varieties are more cut by mature WSS larvae. Typically, lines that are more WSS-cut are also more attractive because they receive more eggs. Do not plant less attractive wheat around a more attractive crop, the females forage through the unattractive crop exterior to find the attractive crop in the interior.
- Plant a solid-stem winter wheat variety on the outside field border of a spring wheat crop. As in the previous point, adult WSS are more attracted to stems with greater development and growth. Wheat at a later development stage will be more attractive to WSS than wheat at an earlier development stage and can help protect the younger crop.
- Follow pollinator conservation practices to preserve parasitoid populations. There are no current insecticides that can adequately suppress WSS. However, WSS adults are susceptible to some insecticides that may be applied for other insect pests, such as grasshoppers or cutworms. At times, the numbers of dead adults from such treatments can appear impressive, but the benefit is negligible. In addition, the more long-lived parasitoids of WSS are very susceptible to insecticides, with impacts on parasitoids more severe than on the WSS. Avoid the use of insecticides such as pyrethroids, carbamates, or organophosphates to preserve parasitoid populations. It is believed that the use of benzoylphenylurea insecticides, such as diflubenzuron, will not harm adult parasitoids f lying in fields because the mode of action targets immature stages such as nymphal grasshoppers and caterpillars. Therefore, larval WSS, and the developing immature parasitoids feeding on them are protected because they are in the stem interior.
- Provide nectar on the landscape for parasitoids. The original prairie habitat for WSS and its parasitoids included flowering species
interspersed among grasses. Recently, Montana State University research has shown
that adult parasitoids surviving only on nutrient reserves carried over from eating
immature WSS larvae do not live as long as those that also feed on supplemental nutrition,
like floral nectar. However, it can be challenging to provide supplemental nutrition
sources in fieldgrown crops, since parasitoids are quite small and have difficulty
accessing nectar from many commonly planted f lowering cash crop species such as pea,
lentil, canola, and camelina. Alternatively, buckwheat is an excellent nectar source
for parasitoids, but care should be taken to not plant buckwheat in areas where it
could potentially contaminate a wheat crop at harvest (Figure 14). Avoid planting buckwheat immediately adjacent to a wheat crop. USDA-NRCS provides
guidance for excluding buckwheat in conservation plantings and crop rotations with,
or adjacent to, commodity wheat fields that have a scheduled rotation to wheat within
the next two calendar years to avoid potential contamination of the wheat crop for
export to Japan. See USDA-NRCS National Bulletin 190-23-14 for more information (USDA-NRCS,
2023).
Many flowering species will benefit a diverse array of native pollinators, and some will benefit parasitoids, such as safflower, deerhorn clarkia, native sunflowers (not commercial sunflowers), fava bean, and cowpea. Pollinator plantings adjacent to wheat fields will benefit parasitoid populations by providing supplemental nutrition, potentially increasing parasitoid lifespans from as many as 50 days or more and giving greater opportunity for these parasitoids to locate more WSS larvae to parasitize. This is an ongoing research area, and more beneficial plant species may be identified in the future.
Figure 14. Buckwheat (Fagopyrum esculentum) plants in early bloom stage. Photo: USDA-NRCS. - Extend latest wheat harvest dates by planting spring wheat near winter wheat. WSS produces only one generation per season and can easily survive in droughtstressed crops. Parasitoids produce two generations per season and both generations require nutrients they acquire from WSS larvae that are still active in ripening wheat stems. Placing spring wheat near winter wheat will provide additional larval food sources for parasitoids due to the later ripening of spring wheat. Early harvest of wheat due to drought collapses parasitoid populations without directly impacting WSS, resulting in more damaging WSS numbers in crops that follow one or more years of drought.
- Conserve smooth brome near wheat fields for parasitoid habitat. Before 1920, smooth brome was identified as a potential trap crop for WSS due to
many larvae dying while feeding in the stems. Smooth brome is often seen in areas
adjacent to wheat fields due to historic use in pastures or for erosion control after
disturbance. Due to its late ripening and relatively high levels of immature WSS mortality,
it serves as a sink that limits WSS populations while providing some active WSS larvae
for second-generation parasitoids to consume long after wheat harvest. Conserve existing
smooth brome areas to serve as parasitoid reservoirs, especially when summer rainfall
is limited (Figure 15). Other grasses like western wheatgrass could also serve as reservoirs for parasitoids
but are also highly susceptible to WSS and could thus also be considered pest reservoirs.

Figure 15. Conserve smooth brome (Bromus inermis) stands adjacent to wheat fields to serve as parasitoid reservoirs. Photo: M. Lavin, MSU. - Do not rely on swathing to control WSS. In areas with severe WSS stem cutting, serious crop loss can be avoided by using a swather to windrow the wheat before combining. However, swathing will not kill even some of the WSS larvae inside the stem unless the standing wheat is quite unripe, which results in loss of grain quality. WSS larvae are located higher in the stems when the wheat is unripe. In contrast, when the wheat is mature, the WSS larvae have migrated down the stem to at or below the soil surface and are located below the swather cutting height. Swathing mature wheat only serves to destroy parasitoid habitat. If swathing, cut as high as possible (minimum of one-third of the total plant height) to maintain parasitoid habitat. Swathing alone will also not protect from yield loss. In addition, because swathing is harmful to parasitoids, this practice results in larger WSS populations and increased crop loss over time.
- Know the cost of WSS damage and swathing. Producers are encouraged to do a partial-budget analysis of the ownership and operating costs of swathing equipment. Producers in areas with high WSS infestation who continue to plant the highest-yielding hollow-stem wheat variety with the intent to swath are likely seeing decreasing profitability due to the costs of owning both a swather and a combine and the potential need for two machinery passes at harvest. When these costs are added together with decreased wheat yield due to WSS, the long-term effect of swathing results in a protracted loss to the bottom line. A good partial budget comparison helps make this loss more visible to the producer. Producers may only be looking at the acceptable yields in these imperfect swathing approaches, not realizing their profitability could be much greater by following a complete package of best management practices.
References
Achhami, B.B., G.V. Reddy, J.D. Sherman, R.K.D. Peterson, and D.K. Weaver. 2020. Antixenosis, Antibiosis, and Potential Yield Compensatory Response in Barley Cultivars Exposed to Wheat Stem Sawfly (Hymenoptera: Cephidae) under Field Conditions. Journal of Insect Science 20(5):114. Open access: https://doi.org/10.1093/jisesa/ieaa091
Fulbright, J.L., K.W. Wanner, A. Bekkerman, and D.K. Weaver. 2017. Wheat stem sawfly biology- updated. Montana State University Extension MontGuide MT201107AG, Montana State University, Bozeman, MT. 4p.
Robertson, H.M., R.M. Waterhouse, K.K. Walden, L. Ruzzante, M. Reijinders, B.S. Coates, F. Legeai, J.C. Gress, S. Biyiklioglu, D.K. Weaver, K.W. Wanner, and H. Budak. 2018. Genome Sequence of the Wheat Stem Sawfly, Cephus cinctus, Representing and Early-Branching Lineage of the Hymenoptera, Illuminates Evolution of Hymenopteran Chemoreceptors. Genome Biology and Evolution 10(11):2997-3011. Open access: https://doi.org/10.1093/gbe/evy232
USDA-NRCS. 2023. National Bulletin 190-23-14 ECS. Excluding Buckwheat in Conservation Plantings In or Near Commodity Wheat Fields in Selected West and Central States. USDA-NRCS Title 190- Ecological Sciences National Bulletins. Available at: https://directives.sc.egov.usda.gov/landingpage/35172
Weaver, D.K. 2022. Ecological Considerations from a Prairie Origin Inform Wheat Stem Sawfly Management. Montana Institute on Ecosystems Rough Cut Seminar Series: Webinar. Montana State University, Bozeman, MT. Available at: Ecological Considerations from a Prairie Origin Inform Wheat Stem Sawfly Management
Weaver, D.K. 2023. Chapter 5: Wheat stem sawfly, Cephus cinctus. In S.D. Eigenbrode and A. Rashed (eds.), Advances in Understanding Insect Pests Affecting Wheat and Other Cereals, Burleigh Dodds Science Publishing, Cambridge, UK. p. 93 – 134. Open access: https://www.bdschapters.com/_webedit/uploadedfiles/All%20Files/Open%20Access/9781801468435.pdf
Copyright © 2024 MSU Extension
We encourage the use of this document for nonprofit educational purposes. This document may be reprinted for nonprofit educational purposes if no endorsement of a commercial product, service or company is stated or implied, and if appropriate credit is given to the author and MSU Extension. This publication is available electronically (free) and in hard copy from Montana State University Extension at montana.edu/extension/ and USDA-NRCS Montana at nrcs.usda.gov/montana.
The U.S. Department of Agriculture (USDA), Montana State University and Montana State University Extension prohibit discrimination in all of their programs and activities on the basis of race, color, national origin, gender, religion, age, disability, political beliefs, sexual orientation, and marital and family status. Issued in furtherance of cooperative extension work in agriculture and home economics, acts of May 8 and June 30, 1914, in cooperation with the U.S. Department of Agriculture, Cody Stone, Director of Extension, Montana State University, Bozeman, MT 59717.
Acknowledgments
The authors thank Kevin W. Wanner for early contributions to this document. Research conducted by Montana State University was supported by: Montana Wheat and Barley Committee, Montana Grains Foundation, USDA CSREES Special Research Grants, USDA NIFA Foundational Grants, Montana Board of Research and Commercialization Technology, Montana Research and Development Initiative, BNSF Railway Foundation, Montana Seed Trade Association, Cahill Seeds, Pro Co-op Ag Center, and many private ag producers who have tirelessly aided research efforts.
The mention of research sponsors does not constitute an endorsement by USDA-NRCS.
Non-discrimination Statement
In accordance with Federal civil rights law and U.S. Department of Agriculture (USDA) civil rights regulations and policies, the USDA, its agencies, offices, employees, and institutions participating in or administering USDA programs are prohibited from discriminating based on race, color, national origin, religion, sex, gender identity (including gender expression), sexual orientation, disability, age, marital status, family/parental status, income derived from a public assistance program, political beliefs, or reprisal or retaliation for prior civil rights activity, in any program or activity conducted or funded by USDA (not all bases apply to all programs). Remedies and complaint filing deadlines vary by program or incident.
Persons with disabilities who require alternative means of communication for program information (e.g., Braille, large print, audiotape, American Sign Language, etc.) should contact the responsible Agency or USDA’s TARGET Center at (202) 720-2600 (voice and TTY) or contact USDA through the Federal Relay Service at (800) 877-8339. Additionally, program information may be made available in languages other than English.
To file a program discrimination complaint, complete the USDA Program Discrimination Complaint Form, AD-3027, found online at How to File a Program Discrimination Complaint and at any USDA office, or write a letter addressed to USDA and provide in the letter all of the information requested in the form. To request a copy of the complaint form, call (866) 632-9992. Submit your completed form or letter to USDA by (1) mail: U.S. Department of Agriculture, Office of the Assistant Secretary for Civil Rights, 1400 Independence Avenue, SW, Washington, D.C. 20250-9410; (2) fax: (202) 690-7442; or (3) email: [email protected].
USDA is an equal opportunity provider, employer, and lender.
