Montana IPM Bulletin, Spring 2018

The Montana IPM Bulletin presents critical pest management and pesticide education articles for Montana homeowners, pesticide applicators, farmers and ranchers. These articles are designed to deliver timely updates from an unbiased perspective that are specific to Montana. This is a cooperative effort between Montana State University pesticide education and integrated pest management programs.
Contents
- Sticky Barriers Protect Urban Trees from Aphids
- New Resources from Montana State University’s IPM Program
- Look for Ventenata this Summer
- Montana State University, the next host institution of the Western Region Sustainable Agriculture Research and Education (SARE) Program
- Ask the Expert
- Pest Management Toolkit
- Meet Your Specialist
- Acknowledgments
PDF links can be viewed with Adobe Acrobat or Sumatra PDF.
Sticky Barriers Protect Urban Trees from Aphids
Ruth O’Neill, Research Associate, MSU Dept. of Plant Sciences and Plant Pathology

Ventenata. Photo by Inna Smith.
In the early spring on a warm day, just as leaf buds are breaking, you may see numerous ants running up tree trunks, investigating twigs and buds. Look closely, and you may see tiny, newly-hatched aphids running along the trunks and twigs as well. If the leaves are already unfolding, you may see aphids, singly or in clusters, feeding on the undersides of new leaves, with ants lingering near them.
What is going on?
The Problem: Aphids are fragile, sap-feeding insects with straw-like mouthparts. They
cause serious leaf damage to many garden flowers, vegetables, shrubs, and trees (Figure 1), and they can vector certain plant diseases. Aphids also excrete a large volume
of excess plant sap in their waste or “honeydew,” which is a nuisance for homeowners
when it coats sidewalks, cars, and anything else outside. Plus, honeydew provides
a sugary growth medium for black sooty mold, which is unsightly.
However, honeydew is also a valuable food source for many animals. This is particularly
true of ants, who collect the droplets of honeydew directly from the aphids’ back
ends, before it ever hits the ground (Figure 2). Diverse species of ants and aphids (and some of their relatives, like scale insects)
have evolved simple or complex mutualistic relationships, with ants herding the aphids,
and guarding them from their natural enemies in exchange for the honeydew reward.
It’s a cooperative relationship, benefiting both sides.
Aphids are prolific reproducers in the spring, even skipping the normal insect egg
stage to speed matters up. Instead they give live birth to female clones, who will
themselves become aphid-clone-making machines, at a rate of about five births per
day in the spring. But aphids have many common and widespread natural enemies that
curb their numbers, including damsel bugs, lacewing larvae (a.k.a. ant lions), hover
fly larvae, lady beetles (both larvae and adults), big-eyed bugs, spiders, yellowjackets,
hornets, and parasitoid wasps. Tiny Pemphredon wasps paralyze aphids and pack them
into their nests for their larvae to feed on.

In the absence of their ant bodyguards, aphids are usually well-controlled by these natural enemies. When ants are present, however, they patrol twigs and leaves very persistently for intruders – just test this out by sticking a finger near one of their aphid colonies and you may get bitten by an ant. The ants attack beneficial aphid-hunters, and their livestock thrive and multiply (Figure 3).


FIGURE 3. Ants locate a feeding lady beetle and attack it, biting between the body and leg segments to drive it away.
A Highly Effective Solution
For trees, there is a highly effective solution to an aphid problem: Tanglefoot barriers around the trunks (Figure 4). Tanglefoot is a viscous, sticky petroleum product, available at hardware stores. Note that it is NOT a good idea to apply Tanglefoot directly to a tree trunk, because it stays on there more or less permanently, disfiguring the tree. Tanglefoot can also damage the bark, making it swell and crack.
-
Pros:

- Effective, economical
- Circumvents pesticide use
- Suppresses ant colony size over time
- Cons:
- Messy to apply
- Can rub-off on pets/kids
Here are the materials to make a Tanglefoot band:
- Tanglefoot, either in a tube or a tub.
- Polyester fiber fill. This can be purchased as quilt batting at fabric stores.
- A paper band, made of stiff water-resistant paper (e.g., card stock). This needs to be at least six inches tall and wide enough to reach around the tree and overlap by several inches.
- Popsicle stick or twig (if not using Tanglefoot from a tube).
- Long twist ties or zip-ties.
How to apply a Tanglefoot barrier:
- Pick a place on the trunk that is as high as possible, just below the first branches, to keep pets from rubbing against the Tanglefoot.
- Wind a long strip of the fiber fill, roughly the same height as the paper band, around
the tree a few times. Polyfill is better than cotton, because cotton can get soggy
in wet weather. This layer will fill all of the irregularities in the bark, so that
the ants can't crawl underneath the band. It also gives the trunk a little growing
room, so that the band will not pop of or impinge on tree growth.
- Wrap the paper band over the fiber fill and secure it at the top and the bottom with
the twist ties or zip-ties.
- Use the popsicle stick or twig to apply a band of Tanglefoot just below the top edge of the paper band (the product tends to sag a little in hot weather). Make the band two or three inches tall and about 1/8-inch thick.
You will see immediate results, as huge numbers of ants build up on either side of
the barrier, unable to cross over it.
Ants imprisoned above the barrier will eventually jump off the tree. Within a week,
you should see new, undamaged growth emerging on trees.
Over time dirt, and insects will stick to the surface of the Tanglefoot, and ants
will be able to walk on it. Once a week or so, spread the Tanglefoot around to refresh
the surface and add a little more if needed.
New Resources from Montana State University’s IPM Program
Sarah Eilers, MSU IPM Manager
The Montana State University Integrated Pest Management (IPM) Program has created a communication network that allows Montanans to find IPM-related resources in a manner that best suits their needs. Outreach to farmers, ranchers, green industry professionals, pesticide applicators and homeowners can be challenging due to Montana’s long distances, limited access to technology, and scarcity of specialists. By relying on various innovative methods of outreach, the IPM Program has been able to reach thousands of stakeholders across the state.
The Schutter Diagnostic Lab, where specialists provide clients with diagnostic reports that identify disease, insects, weeds, and other plant issues, is an important part of the IPM Program. The lab averages 2000 samples per year from both agricultural and residential environments. Integrated pest management tactics, along with traditional pesticide recommendations, are disclosed in final diagnostic reports.
Urban and Ag Alerts are other important components of the communication network between campus specialists and diverse constituents in Montana. Alerts are time-sensitive documents that are released when trends in pest occurrences are found in sample submissions to the Schutter Diagnostic Lab or via other communication with specialists like face-to-face meetings, texts, emails, and phone calls. Alerts are distributed via e-mail, text, and fax. These notifications allow specialists to communicate information about emerging issues with over 1,500 subscribers.
Updates from the IPM Program can also be obtained through social media platforms such as Facebook and Twitter. As more and more people rely on smart phones to obtain information, and with improved cell phone coverage to rural areas, social media has become a significant outreach tool and a good way to interact with clients.
The newest addition to the outreach toolbox is digital diagnosis. The Plant Sample Submission app was introduced in the fall of 2017. The app is available on both iOS and Android platforms. Users can submit digital photo samples. Disease, Insect and Plant identification categories are available on the app. The app walks clients through submission forms and questions that relate directly to specific types of situations. For instance, vegetables, turf and lawn, and feld/agronomic crops are three land use categories to choose from in the app. The app is designed for the person on-the-go. For more information about downloading and using the app, see the Schutter Diagnostic Lab website.
Montana Ag Live is one of the more entertaining outreach activities of the IPM Program. It is presented by Montana PBS in association with MSU Extension and other program sponsors. It is a live television program that covers relevant agricultural and horticultural topics specific to Montana. Programs are aired on Sunday evenings at 6 p.m. in the spring and fall. The program includes a panel of specialists who answer callers’ questions and give recommendations on best practices. The average viewership is 10,000 viewers per episode.
The annual Pest Management Tour is a highly valued educational opportunity sponsored by the MSU Pesticide Education Program (PEP) and the IPM Program. It is designed for approximately 5,500 private applicators across the state. The 4-5 day tour focuses on one of five Montana regions each year, with each region made up of multiple counties. The program features presentations from MSU Extension specialists and local Extension agents who highlight research and management techniques. Areas of focus are in calibration techniques, cropland insect pests, plant disease management, herbicide resistance, forage pest management, and cropland and rangeland weeds.
The most meaningful resource of the IPM Program’s communication network is the rapport between specialists and stakeholders. Extensive collaborations with professional organizations along with state and federal agencies have been integral to the success of the IPM Program. Personal relationships and experience with stakeholder groups have driven the priorities of the IPM Group. Feedback from stakeholders helps structure outreach and research needs.
The small population of Montana lends itself to collaboration. These relationships throughout the state allow for this communication network to spread the word on reducing health and environmental risks from pest management, improving IPM practices and increasing IPM adoption.
For more information about the IPM Program at MSU, please see our website.
Look for Ventenata this Summer
by Jane Mangold, Extension Invasive Plant Specialist; and Audrey Harvey, Graduate Research Assistant
Just when you thought cheatgrass was the only invasive annual grass to worry about on Montana range and pasture land, along comes ventenata (Ventenata dubia). Ventenata is a winter annual grass native to southern Europe, western Asia and northern Africa. It is also known as wiregrass or North Africa grass. The first reports of ventenata in North America were in the 1950s in northern Idaho. Over the last few years, ventenata has been increasing quickly on rangeland and improved pastures in western Montana, especially in Gallatin, Lake, Missoula, Ravalli, and Sanders counties. Ventenata is easily mistaken for cheatgrass (Bromus tectorum) or Japanese brome (B. japonicus). As a new invader in Montana, be on the lookout for ventenata this summer.

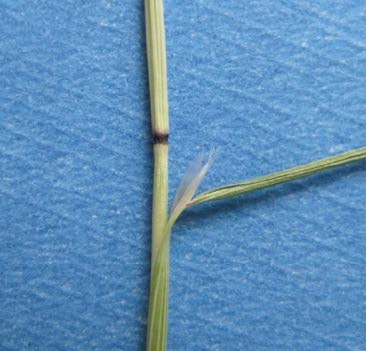
FIGURE 6. The nodes of ventenata are reddish to black in color, and the ligule is membranous and unusually long. Photo by Tim Prather, University of Idaho.

Ventenata is typically 6-27 inches tall, with leaves that are rolled lengthways or folded. It has open sheaths, and the inflorescence is open and pyramidal in shape (Figure 5). The color of ventenata has been described as tawny to light yellow. Grass identification can be difficult for many people, so look for these key characteristics on ventenata: reddish to black nodes in May-June (Figure 6); an unusually long, membranous ligule (1-8mm) (Figure 6); distinct shiny appearance and open panicle in June-July; and lower awns that are straight and upper awns that are twisted and bent once the plant senesces in July-August (Figure 7).
Ventenata has been found along roadsides and in hay, pasture, range and CRP fields in the western U.S., particularly northeastern Oregon, northern Idaho, eastern Washington, and most recently in western Montana. Ventenata is most common on south-facing hillsides with shallow, rocky, clay or clay-loam soils. As an annual, the plant reproduces only by seed, and spread occurs primarily through contaminated grass seed, hay and annual crops. The long awns likely assist in dispersal by sticking to humans and animals.
Ventenata is a state-listed noxious weed in Utah and Washington and a county-listed noxious weed in some counties in northcentral Wyoming. Information on management is limited, especially for range and pasture settings. First and foremost, maintaining healthy grassland plant communities and limiting disturbance can help to prevent ventenata from establishing. Mowing multiple times during the growing season can be effective. However, mowing once during heading does not work, as the plant bends over and becomes tangled in the mower, and plants mowed prior to heading may produce another flush of seeds if soil moisture is adequate. Ventenata has flourished following fire in Oregon; in Idaho, fire suppressed ventenata but tended to stimulate annual weedy bromes and left an opening for more ventenata the following year. The plant is unpalatable once panicles appear, so grazing as a management option is limited to early spring. Herbicides labeled for control of ventenata in pasture, range and CRP are limited because ventenata is such a recent issue. However, last fall ventenata was added to the Esplanade label (active ingredient indaziflam) for control in rights-of-way and natural areas. Research from Idaho showed imazapic (e.g. Plateau or Panoramic 2SL) to be effective when it was applied in the fall to semi-dormant perennial grass stands, particularly when ventenata comprised more than 25% of vegetative cover. Trials in Montana suggest indaziflam may provide at least two years of control, but results are still preliminary.
Land managers in Montana are trying to understand the distribution of ventenata across the state. Identifying the invasive grass when patches are still small is very important for early detection and rapid response. Take note of the identifying features and photos discussed in this article.
If you think you see ventenata while in the field this summer, please contact your local Extension or weed district office, the Schutter Diagnostic Lab, or Jane Mangold
Montana State University, the next host institution of the Western Region Sustainable Agriculture Research and Education (SARE) Program
Fabian Menalled, Extension Cropland Weed Specialist
Functioning in 50 states and supported through competitive grants that are conducted cooperatively by farmers, ranchers, researchers and agriculture professionals, the Sustainable Agriculture Research and Education (SARE) program’s mission is to advance sustainable agriculture that is profitable, environmentally sound, and good for communities. Since 1988, SARE, a program of the U.S.Department of Agriculture, has funded more than 5,000 groundbreaking research and education projects. SARE defines sustainable agriculture as agriculture that is economically viable, socially supportive and ecologically sound. It facilitates on-farm research innovations led by producers and multidisciplinary teams of experts. Western SARE is one of four regions organized under the national SARE and encompasses 13 western states as well as American Samoa, the Northern Mariana Islands, Micronesia and Guam.
Beginning September 1, 2018, and with an annual grant and operational budget of more than $8 million, MSU will coordinate during the next five years the Western SARE program while administering four of its five competitive grant programs. This includes: research and education grants, farmer-rancher grants, professional and producer grants, and graduate student grants. The University of Wyoming will administer Western SARE’s professional development grant program.
Fabian Menalled, professor of weed ecology and management in the Department of Land Resources and Environmental Sciences, has been named the Western SARE regional coordinator. Since 2012, Menalled has been Montana’s state SARE coordinator, a position that requires him to promote sustainable agriculture locally through noncompetitive grants operated through SARE. Shelley Mills, MSU Extension agent in Valley County, has been selected as the new state coordinator.
As the regional coordinator, Menalled will support Western SARE’s administrative council, a governing board composed of farmers and ranchers along with representatives from universities, government, agribusinesses and non-profits. Montana has two agricultural producer representatives on the Western SARE Administrative Council: Kent Wasson, farmer and rancher in northern Phillips County, and Anna Merriman, who owns 4M Farms near Malta. The regional council sets guidelines, develops calls for proposals, and establishes project priorities and ensures funded projects have the appropriate balance and diversity that can represent the region’s wide- ranging geography and subject matter.
Utah State University hosted the Western SARE program since 1991. The decision to appoint MSU as the next host institution was based on a competitive process in which universities across the region presented proposals to host the program for a five-year period. The goal of the MSU-hosted Western SARE program is to increase the program’s focus on including minority populations and women in the Western region, in addition to supporting and training the next generation of farmers and food producers.
For more information about the Western SARE program, please contact Fabian Menalled
Ask the Expert
Q. Have you conducted or seen any studies about repeated mowing to control western salsify?
Jane Mangold says: I have not initiated or observed any studies about repeated mowing to manage salsify, but I think it’s worth a try. Research on mowing to control other species that reproduce by seed only, such as spotted knapweed, has shown it can be effective if mowed just prior to or at time of flowering for 2-3 years. The key is timing. Don’t mow too early or it will grow low and still flower. Instead, you want to mow just before the flower buds open or shortly after they open, well before seed formation. This is typically between late May and mid-June for western salsify. You should be prepared to mow again when the mowed plants grow back and try to flower again. One of the challenges of managing salsify is its wind-dispersed seed. If there is a lot of western salsify on neighboring lands that isn't being controlled, seeds will blow onto your property regardless of your attempts to control it. So, the chance for long-term success via mowing is less than it might be for a species like spotted knapweed whose seeds don’t blow around as much.
Q. I’m growing a specialty crop and there aren’t many conventional or biopesticides labelled for use. Why is this?
Mary Burrows says: Traditionally, there has been limited research by pesticide companies for specialty crops primarily due to the wide range of crops that need to be tested and the limited profit potential for crops grown on limited acres. Each crop needs to be tested for efficacy of the product, crop safety, and the amount of pesticide residue left on the crop at harvest. In 1963, Land-grant universities recognized the critical need to include specialty crops on pesticide labels. The Inter-regional Project #4 (IR-4) has been a major resource for developing and supporting these registrations for over 50 years. In Montana some of these specialty crops include mint, camelina, sugarbeet, potato, malt barley, cherries, apples, safflower, and sunflower. If you are looking for biopesticides to use on your crop, consult the IR-4 biopesticide and organic support database, it’s a great resource that I use routinely.
Lately my role as the IR-4 representative in Montana has kept me busy. I’ve received a request to work with Canada on herbicide residue limits in coriander. I keep a list of growers that have grown strange crops for Montana such as coriander, and even quinoa, chia, industrial hemp, and mint. I called a grower who had grown coriander last year (his grandpa had grown it, and he tried it last year). Turns out he has all the pesticides he needed. So this particular issue will be a low priority for Montana, but it’s an interesting part of my job keeping up on all the specialty crops, and it certainly presents routine challenges. “The next time you drive by a field of a specialty crop like crambe (Figure 8) (an oil seed crop), think of IR-4!
Q. I have a lot of wild oat in my spring wheat this year and herbicide is not an option. What can I do?
Tim Seipel says: Weed management requires a long-term strategy and an integrated approach, sometimes referred to as ‘many little hammers’. Such a strategy involves diverse crop rotations to disrupt the weed’s life cycle (i.e spring versus winter wheat) and should include competitive crops to limit the weed’s impact. Based on results from a study at MSU’s Post Farm in Bozeman, last year we found that seeding spring wheat at 90 lbs/ac reduced yield loss to wild oat, and wild oat performance was worse compared to when spring wheat was sown at 60 lbs/ac. Additionally, yield loss was lower when tall varieties were grown, and performance of wild oat was worse because it didn't extend above the wheat canopy. While it is no silver bullet, integrating tactics can limit yield loss to weeds and slowly, over time, reduce the weed’s seedbank.
Q. Is an individual who doesn't apply pesticides required to follow Worker Protection Standards (WPS) if contracting a commercial pesticide applicator?
Cecil Tharp says: Yes. The 1992 and updated 2015 WPS is meant to protect agricultural workers and handlers from pesticides. The responsibility of protecting workers or handlers falls on the owners of the agricultural establishments, regardless of whether the owner applies pesticides or not. Owners must follow WPS if these two items apply:
- pesticide application is for the purpose of managing an agricultural commodity on an agricultural site
- the pesticide has an agricultural use requirements box
Limited WPS requirements apply even if an owner does not have workers (owner must ensure all family members stay out of the field for a period of time after application, otherwise known as the re-entry-interval; owner must ensure all family members stay out of application exclusion zone; owner must follow the entire ag-use requirements box on the label).
If non-family member workers are present, the requirements include annual WPS training for workers, providing a central posting area, providing decontamination sites, displaying WPS safety posters, communicating with workers regarding applications, occasionally placarding entry points to fields, implementing the application exclusion zone (AEZ) and following the re-entry interval on the pesticide product label. It is the commercial applicator’s responsibility to share WPS information with the owner of the agricultural establishment, however it is the owner’s responsibility to ensure workers are protected.
See the Pesticide Education Program website for all WPS requirements.
Pest Management Toolkit
- New Extension fact sheet on common buckthorn (MT201708AG), a species added to the Montana noxious weed list in 2017. Common buckthorn is a woody shrub or small tree growing 6-25’ tall that invades uplands, grasslands, and disturbed areas, forming dense thickets that may crowd or shade out native plants. 720KB PDF, color, 2 pages.
- New MontGuide available: Fungicide use in Field Crops: Classification, Risks, Use, and Economics (MT201705AG). Fungicides are effective tools for disease management. This Montguide covers when
fungicides could be used, what types of fungicides are available, the risk of fungicide
resistance, where to find data on fungicide efficacy, and the economics of fungicide
use in wheat. 650KB PDF, color, 8 pages.
- Worker Protection Standard Training.For owners of agricultural establishments, private pesticide applicators, workers,
and handlers. Contact Amy Bowser ([email protected]) or check the online agenda for more information. Private applicator credits will be available. Dates:
- Glasgow on May 1
- Great Falls on May 2
- Kalispell on May 3
- Level 1 Noxious Weed Management Course, September 11-13, 2018, Bozeman. For more details go to the Invasive Plants Workshops page.
- 2018 Pest Management Tour. Across PAT District 1 in Lincoln, Flathead, Sanders, Mineral, Missoula, Lake, Ravalli Counties. October 1st-5th. Six private applicator credits. This program will include pest management updates over a variety of topics. Agenda and registration information will be available by July 1 at [link has expired] or contact Cecil Tharp for more information.
Meet Your Specialist
Bright Agindotan, Regional Pulse Crop Diagnostic Laboratory Manager and Assistant Research Professor, Montana State University, Bozeman

What is your field of interest (scholastic and research)?
My fields of interest are plant virus discovery, plant pathogen detection, and plant disease management.
When did you arrive in Bozeman?
I arrived in Bozeman on September 14, 2014.
Where are you from originally?
I was born in Sapele, Nigeria, and grew up in Lagos, where I had all my education up to my first degree. Lagos, Nigeria is a city of 21 million people. I attended Baptist Academy, Lagos (founded in 1855), a premier secondary school in Nigeria.
What do you like to do in your spare time? Any hobbies?
Bible studies, watching soccer, following global events, watching Nigerian movies in YouTube, and jogging.
What are some important areas of focus in your field?
Identification of pathogens of pulse crops (chickpea, dry peas, and lentil) and the management of their diseases.
Describe some past research projects:
At Cornell University, I developed a membrane-based macroarray for the detection of viruses and viroids of solanaceous crops.
At the University of Illinois, I developed a sequence-independent amplification method coupled with next generation sequencing, which I used to detect nine new viruses in a bioenergy crop, switchgrass (Panicum spp).
Where/when did you receive your degrees?
My bachelor’s in biochemistry was in 1987 from the University of Lagos, Nigeria; master’s in biotechnology in 1997 from the Federal University of Technology, Owerri, Nigeria; doctorate degree in biochemistry in 2001 from the University of Ibadan, Nigeria. I then earned an MBA degree from the University of Illinois in 2007.
Where have you worked/taught in the past?
I had worked at the following universities as a postdoctoral research associate or research associate: University of Idaho, Moscow (2001- 2003); Kansas State University, Manhattan (2003-2004); Cornell University, Ithaca (2004-2006); and at the University of Illinois, Urbana-Champaign (2007-2014).
At the University of Idaho, I developed a multiplex real time RT-PCR detection system for viruses of potatoes.
What are some of your current projects?
My current projects include the survey of Montana for dry pea diseases, characterization of pathogens detected during our dry pea field surveys, and the evaluation of the eficacy of some fungicides for seed treatment.
Acknowledgments
Do You Have a Question or Comment Regarding the Montana IPM Bulletin?
Send inquiries and suggestions to:
Cecil Tharp
Pesticide Education Specialist
P.O. Box 172900
Montana State University
Bozeman, MT 59717-00
Phone: (406) 994-5067
Fax: (406) 994-5589
Email: [email protected]
Web: pesticides.montana.edu
Jane Mangold
Invasive Plant Specialist
P.O. Box 173120
Montana State University
Bozeman, MT 59717-3120
Phone: (406) 994-5513
Fax: (406) 994-3933
Email: [email protected]
Web: landresources.montana.edu
Noelle Orloff
Associate Extension Specialist
P.O. Box 173120
Montana State University
Bozeman, MT 59717-3120
Phone: (406) 994-6297
Fax: (406) 994-3933
Email: [email protected]
Web: diagnostics.montana.edu

Common chemical and trade names are used in this publication for clarity by the reader. Inclusion of a common chemical or trade name does not imply endorsement of that particular product or brand of herbicide. Recommendations are not meant to replace those provided in the label. Consult the label prior to any application.
Original Spring 2018 PDF (1.4MB)


