MSU Biosafety Manual
Updated: September 2023
Interim Biosafety Officer: Amy Robison, [email protected] (406) 994-6733
MSU Office of Research Compliance
- TABLE OF CONTENTS
- Chapter 1 Biological Safety Program: Purpose, Scope, Responsibilities
- Chapter 2 IBC Approval of Research Projects
- Chapter 3 Regulatory Guidelines
- Chapter 4 Biohazardous Materials
- Chapter 5 Routes of Transmission
- Chapter 6 Biosafety Principles
- Chapter 7 Biosafety Levels
- Chapter 8 Laboratory Biosafety Practices
- Chapter 9 Engineering Controls
- Chapter 10 Personal Protective Equipment (PPE)
- Chapter 11 Training for Laboratory Personnel
- Chapter 12 Decontamination and Sterilization
- Chapter 13 Biohazardous Spill Response
- Chapter 14 Biohazardous Waste Disposal
- Chapter 15 Transportation and Shipment of Biological Materials
- Appendix A Importation and Exportation of Etiologic Agents
- Appendix B Laboratory Ventilation and Containment for Biosafety
- Appendix C Biosafety Level 2 (BSL-2) Requirements
Chapter 1- Biological Safety Program: Purpose, Scope, Responsibilities
Purpose
The purpose of this Biosafety Manual is to define policies and procedures pertaining to the use of biological materials in research laboratories at Montana State University (MSU). These policies and procedures are designed to safeguard MSU researchers, students, community, and environment from biological hazards with minimal impact on research.
The work practices, procedures, and policies specified in this manual are based on regulatory requirements and accepted biosafety practices. Implementation of these measures will reduce the likelihood that an incident involving a biological agent will occur and will fulfill regulatory biosafety requirements. Laboratory work involves potential exposure to biological hazards, as well as to chemical and radiological hazards. Consequently, this manual should be used in conjunction with the MSU Chemical Hazard Communications Plan and Radiation Safety Manual, respectively.
For information about specific biological safety programs and for operations not covered in this manual, contact the Biosafety Officer (BSO).
Scope
This manual applies to all MSU research activities involving biological agents. All faculty, staff, students, and visitors who work on MSU sponsored projects or at MSU facilities are included in the scope of this manual.
Biohazardous materials include, but are not limited to, bacteria; viruses; human or primate tissues, fluids, cells, or cell cultures; wastewater; plant pests and pathogens; plant products and soil.
Responsibilities
Success of the Biosafety Program requires a collaborative effort involving the Biosafety Officer, Institutional Biosafety Committee (IBC), Principal Investigators (PIs), laboratory workers, the Occupational Health Program, and Safety and Risk Management (SRM). PIs are responsible for the health and safety of personnel who work under their supervision and occupy their laboratory space. MSU administration and the IBC endorse this manual and encourage active participation in maintaining high biosafety standards in our research facilities.
Director of Research Compliance (IO)
The IO’s responsibility include:
- Oversight of biohazardous material usage in MSU research through a comprehensive biosafety program which outlines all aspects of dealing with such materials.
- Direct functional responsibility for the IBC and Biosafety Program.
- Effective communication between the IBC and other research related committees on campus.
- Appointment of committee members, in consultation with IBC chairperson.
Institutional Biosafety Committee (IBC)
The Institutional Biosafety Committee (IBC) is responsible for reviewing and approving
research protocols involving recombinant/synthetic nucleic acids, and other biohazardous
materials as outlined in the IBC Manual. The IBC is comprised of faculty representatives
from various academic disciplines, researchers, non-scientific members, students,
and community representatives who are not affiliated with MSU. The IBC typically meets
monthly to review research and other activities submitted to the IBC. The IBC carries
out these functions pursuant to requirements set forth by the National Institutes
of Health (NIH), the Centers for Disease Control and Prevention (CDC), US Department
of Agriculture (USDA), and Occupational Safety and Health Administration (OSHA).
The IBC’s responsibilities include:
- Oversight of MSU’s Biosafety Program, including development of new, and review of existing policies and procedures designed to enhance the Biosafety Program.
- Review and approval of training programs.
- Coordination of biological safety requirements with other campus-wide committees (e.g., IACUC) and programs (e.g., Occupational Health Program).
- Review and approval of new research protocols and their modifications, involving rDNA and biohazardous material in accordance with guidelines established by the OSHA, USDA, CDC, NIH, and MSU policies.
- Define containment levels of biohazardous agents for research projects. Generally, biosafety levels (BSL) established by the CDC and NIH will be used as the level of containment; however, the IBC has the authority to increase or decrease the level of containment according to the project’s specific circumstances.
- Investigation of biosafety violations as well as significant accidents or illnesses involving biological agents.
- If appropriate, the IBC recommends disciplinary action to the appropriate MSU officials.
Biosafety Officer (BSO)
The BSO is responsible for developing, leading, directing, and managing a comprehensive biosafety program for MSU. The biosafety program must meet NIH, CDC, USDA, OSHA, any other granting agency, as well as federal, state, and local requirements. The program includes close cooperation and interaction with committees approving research protocols (IBC), procedures involving human subjects (Institutional Review Board (IRB)), Institutional Animal Care and Use Committee (IACUC), Agricultural Animal Care and Use Committee (AACUC), and Radiation Safety Committee (RSC). The BSO will also provide guidance and consultation to assess the risk of working with potentially biohazardous materials. The BSO interacts with the research, teaching, and diagnostic community to inform and ensure compliance with state and federal reporting or audit requirements, and to inspect and correct deficiencies when noted.
BSO’s responsibilities include:
- Inspection of the physical facilities and containment equipment for compliance with general CDC guidelines for Biosafety Level (BSL) and Animal Biosafety Level (ABSL) laboratories for research and diagnostic work using developed laboratory inspection checklists.
- Review of laboratory specific biosafety manuals and standard operating procedures (SOPs) for compliance with guidelines for BSL and ABSL procedures.
- Provides general guidance about health and safety standards.
- Reviews all research proposals presented to the IBC.
- Helps ensure that biohazards, sharps, and glass wastes are properly treated, transported, and disposed of outside of laboratory facilities per applicable state and federal regulations.
- Maintains list of approved biosafety laboratories with inspection dates and results.
- Responsible for assisting the PIs in designing appropriate lab-specific biosafety manuals for all activities using potentially biohazardous materials
Principal Investigators (PIs)
The PI is primarily responsible for the people and activities in their laboratories and are responsible for implementing an appropriate biological safety program specific for their research projects. PIs should evaluate research operations, perform risk assessments, develop plans for all research activities accordingly, and ensure strict adherence to biological safety practices and techniques for all work involving potentially biohazardous materials.
The PI’s responsibilities include:
- Notifies the IBC regarding all research involving recombinant/synthetic nucleic acid molecules and/or biohazardous materials, submits an IBC protocol for approval, and adheres to all terms and conditions stipulated by the IBC therein.
- Ensures that all laboratory personnel are properly trained and proficient in the biosafety procedures and experimental techniques needed to ensure safety, as well as the MSU protocols for dealing with accidents and injuries.
- Informs the laboratory staff of the reasons and provisions for any precautionary medical practices advised or requested.
- Ensures that individuals working in the laboratory are experienced and proficient in handling biological agents.
- PI must follow everything in the IBC Manual.
- Makes available to all laboratory personnel the protocols that describe all biohazards and their associated precautions.
- Assumes responsibility for all safety practices, techniques, engineering controls and personal protective equipment (PPE) that are required for any biohazards the laboratory.
- Ensures that laboratory biohazards are effectively communicated to laboratory personnel and controls are in place to minimize risks associated with these hazards.
- Notifies the BSO of all spills or incidents involving recombinant/synthetic nucleic acid molecules.
- Notifies the BSO of any spills or incidents involving biological agents that result in exposure to laboratory personnel or the public, or release to the environment.
- Ensures that biohazardous materials are disposed of according to regulations, as outlined in this manual or specific IBC protocols.
- Ensures that biohazardous materials to be transported are packaged and shipped in accordance with regulations by a properly trained individual appointed by the PI.
Occupational Health Program (OHP)
The Occupational Health Program (OHP) is primarily responsible for establishing and performing appropriate medical surveillance for all personnel performing research or supporting research such as animal care workers, facilities, police, and public safety. Surveillance is required at the time of hire or transfer into the research environment and periodically, depending on the work environment, occupational exposure and risk for each position or job category. OHP is responsible for reporting all biological exposure incidents to the appropriate personnel.
Occupational Health Program’s responsibilities include:
- Coordination with Bridger Occupational Health to provide medical evaluations and surveillance program for individuals.
- Filing and record keeping of Worker’s Compensation reports.
- Oversight of the MSU Respiratory Protection Program.
Laboratory Workers
Laboratory workers are the most critical element in maintaining a safe working environment. Individuals must look out for their own safety and that of their co-workers. If individuals do not follow the MSU and laboratory-specific biosafety practices and procedures in the conduct of their laboratory duties, MSU cannot maintain a safe working environment.
Whoever works in the laboratory in a technical capacity is defined as a laboratory worker, whether the person is a PI, student, intern, visiting scholar, or volunteer.
Laboratory workers responsibilities include:
- Participate in and complete all required training and document proficiency to ensure that they are well-informed and up to date on biosafety responsibilities.
- Fully understand the biological agents and procedures used in the laboratory and the risks associated with exposure.
- Adhere to all laboratory practices, and protocols while complying with all applicable policies, procedures, and guidelines.
- Report to the supervisor or PI all problems, procedural discrepancies, spills, potential exposures, or accidental releases as soon as they occur.
- Complete any necessary medical surveillance.
Chapter 2 – IBC Approval of Research Projects
Research and Activity Requiring Review and Approval from the IBC
The IBC reviews and approves many areas of biologically related activities, including research, teaching, and diagnostic projects.
The IBC defines biohazardous materials to include all infectious organisms that can cause disease in humans, animals, or plants, or have a significant negative environmental or agricultural impact. Any materials that are capable of harboring infectious organisms, such as human or primate tissues, fluids, cells, or cell cultures are considered biohazardous material. Biological toxins are also considered biohazardous material.
The IBC Manual outlines work that requires IBC approval. Biohazardous materials are outlined in Chapter 4.
The planning and implementation of safety protocols to prevent laboratory-acquired infections and to eliminate the spread of contamination must be part of every laboratory’s routine activities and lab specific biosafety manual. No work should be considered so important that it jeopardizes the well-being of the worker or the environment.
The handling of biological agents and recombinant/synthetic nucleic acid materials requires the use of precautionary measures dependent on the agents involved and the procedures performed. It is the purpose of this manual to provide background information and guidelines to be used in conjunction with other resources for the evaluation, containment, and control of potentially biohazardous materials in laboratories.
IBC protocol
A PI applying for IBC approval for research or diagnostic activities needs to submit a completed IBC protocol. The IBC protocol process is conducted in TOPAZ Elements. The PI applying for approval must submit an original protocol for review and approval. The PI is responsible for ensuring that all laboratory workers are listed on an approved IBC protocol and trained prior to working with the infectious organisms and/or biohazardous material. The BSO will act as a resource to assist the PI in the IBC submission and approval process.
A PI applying for approval of teaching activities involving potentially biohazardous material must contact the BSO. The BSO will assist the PI in developing a teaching protocol and determining the appropriate biosafety training for teaching assistants involved in the teaching activities.
Protocol Lifecycle
Requests for Amendments After IBC Approval
All amendments to currently approved research and diagnostics activities are required to have IBC review and approval prior to implementation. Minor changes that do not increase the risk to workers, the community, and/or the environment may be processed and approved by the IBC Chair and/or BSO. Examples of administrative modifications may include the addition of personnel and change of laboratory room (if change is to an equivalent and approved facility).
More significant IBC protocol modifications would include the addition of potentially biohazardous materials or procedures that would likely increase the safety risks to researchers. The PI must submit these modifications to the IBC for review. An IBC modification approval is valid until the end of the original approval period (3 years).
Interim Review
An Interim Review notice serves as a mechanism for the PI to provide an annual update of the research occurring on an IBC protocol. The PI must submit an Interim Review the first and second year after initial approval/renewal of a protocol. The PI is asked to list any updates to the protocol as initially approved (or since the last amendment or renewal notice); changes in laboratory staff working on the project, changes to funding, report of any research-related problems/adverse events, and to provide a summary of the project over the last year.
As an essential part of the annual update process, all lab safety training requirements are verified as up to date.
Renewal
A Renewal Protocol is submitted every three years following initial approval. Renewal protocols are sent to full committee review. During renewal amending changes may be made to the protocol, if work outlined in the protocol has changed.
Chapter 3 - Regulatory Guidelines
Federal, State, and Local Agency Regulations and Guidelines
The following is a summary of regulations and guidelines that cover the use of biological agents:
- Centers for Disease Control and Prevention (CDC) and the National Institutes of Health (NIH): Biosafety in Microbiological and Biomedical Laboratories (BMBL), 6th Edition. This document contains guidelines for microbiological practices, safety equipment, and facilities that constitute the four established biosafety levels. The BMBL is considered the standard for biosafety and is the basis for this manual.
- National Institutes of Health (NIH): Guidelines for Research Involving Recombinant or Synthetic Nucleic Acid Molecules (NIH Guidelines). This document provides guidelines for constructing and handling recombinant/synthetic nucleic acid molecules (e.g., rDNA) and organisms containing these materials. Although these guidelines are not subject to regulatory enforcement, institutions that receive any NIH funding for recombinant/synthetic nucleic acid molecules research are required to comply with these guidelines as a condition of funding. The NIH Guidelines requires that each institution establish an Institutional Biosafety Committee (IBC) with the authority to approve proposed recombinant/synthetic nucleic acid molecules research using the NIH guidelines as the minimum standard.
- Occupational Safety and Health Administration (OSHA): Bloodborne Pathogen Standard. This regulation covers occupational exposure to human blood and other potentially infectious materials, including human tissues, body fluids, and cells. OSHA specifies a combination of engineering controls, work practices, and training to reduce the risk of infection. Personnel potentially exposed to human blood or other potentially infectious materials must be offered immunization against hepatitis B virus (HBV) and receive annual training. Personnel who work with HIV or HBV in a research laboratory must receive additional training and demonstrate proficiency in working with human pathogens.
Select Agent Rule
US Department of Health and Human Services (HHS): 42 CFR Part 73 – Select Agents and Toxins; and the Department of Agriculture’s Animal and Plant Health Inspection Service: 7 CFR Part 331 and 9 CFR Part 121, Possession, Use, and Transfer of Biological Agents and Toxins. These regulations require institutions that possess, use, or transfer certain biological agents and toxins (“select agents”) to be registered and approved by DHHS and/or APHIS.
Over Regulatory Requirements
U.S. Department of Transportation (US DOT) and the International Air Transportation Authority (IATA):
These organizations have strict requirements governing the shipment and transportation of hazardous materials, including biological agents. Chapter 11 provides information on shipping regulations.
Centers for Disease Control and Prevention:
The CDC has established specific regulatory requirements for importation or transportation of etiologic agents, which include a permit application that must be submitted and approved prior to any such importations. Title 42 CFR Part 71 outlines the regulations governing the importation of etiologic agents. See Appendix A for more information.
U.S. Department of Agriculture (USDA), Animal & Plant Health Inspection Service (APHIS), & Veterinary Services (VS):
These agencies regulate the import, export, movement, and release of plants, plant pests, soil, genetically engineered organisms, and biofilms.
These agencies also regulate the importation of animals and animal-derived materials to ensure that exotic animal and poultry diseases are not introduced into the United States. Generally, a USDA veterinary permit is needed for materials derived from animals or exposed to animal-source materials. Materials that require a permit include animal tissues, blood, cells or cell lines of livestock or poultry origin, RNA/DNA extracts, hormones, enzymes, monoclonal antibodies for in vivo use in non-human species, certain polyclonal antibodies, antisera, bulk shipments of test kit reagents, and microorganisms, including bacteria, viruses, protozoa, and fungi.
Principal Investigators must apply for permits and receive the approved permit or letter of no jurisdiction prior to working with or transporting any of the above listed materials.
U.S. Department of Commerce:
The DOC has specific regulatory requirements for exportation of biological materials. These regulations are both agent and country specific and must be followed strictly.
Institutional Biosafety Committee:
The DOC has specific regulatory requirements for exportation of biological materials. These regulations are both agent and country specific and must be followed strictly.
Institutional Biosafety Committee:
The IBC has approved several specific policies and procedures that are incorporated into this document as requirements or have been included as appendices.
Chapter 4 - Biohazardous Materials
Categories
Biohazardous materials includes organisms that can cause disease in humans, animals, or plants; or have a significant negative environmental or agricultural impact. Any materials that are capable of harboring infectious organisms, such as human or primate tissues, fluids, cells, or cell cultures are also considered biohazardous material.
The following is a list of biohazardous materials.
- Infectious organisms (e.g., human, animal, plant, and other).
- Viruses
- Rickettsia
- Chlamydia
- Bacteria
- Fungi
- Parasites
- Prions
- Toxins (bacterial, fungal, plant, etc.)
-
- Staff must follow the MSU Biological Toxin Policy
-
- Human and non-human primate cells. Including cell lines, tissue, blood and potentially infectious fluids.
-
Work with animals/animal tissues or vectors known or suspected to be reservoirs of Risk Group (RG) 2 or 3 infectious agents.
-
Cultured cells (all human or certain animal, including non-human primates) and the potentially infectious agents these cells may contain.
-
Recombinant/synthetic nucleic acid molecules
- Vectors used to carry/introduce recombinant/synthetic material
-
Genetically modified organisms, including, but not limited to:
-
Animals, plants, invertebrates, or other organisms created/used by MSU employees.
-
Transgenic field trials involving any genetically modified organisms that are introduced into the environment, including planting of deregulated items in the field.
-
Field testing of plants engineered to produce pharmaceutical and industrial compounds.
-
Prior to initiating any work with biohazardous material, the BSO must be contacted and an IBC protocol must be submitted and approved prior to initiating any work as outlined above in Chapter 2 and in the IBC Manual.
Risk Groups
The Biosafety in Microbiological and Biomedical Laboratories (BMBL) defines the three primary hazardous characteristics associated with a biological agent as the following:
-
The capability of an agent to infect and cause disease in a susceptible human or animal host;
-
The virulence of an agent as measured by the severity of disease; and
-
The availability of preventive measures and effective treatments for the disease.
By taking the route of transmission of the disease into consideration, a standardized methodology was developed to classify biological agents into four different risk groups. Knowing the Risk Group (RG) of an agent assists researchers and safety professionals in determining the appropriate safety protocols to be followed. The NIH defines RGs and classifies biological agents into their respective RG (NIH Guidelines Appendix B).
Risk Group 1 (RG1)
Agents not associated with disease in healthy adult humans.
Risk Group 2 (RG2)
Agents associated with human diseases that are rarely serious and for which preventive or therapeutic interventions are often available.
Risk Group 3 (RG3)
Agents that are associated with serious or lethal human disease for which preventive or therapeutic interventions may be available (high individual risk but low community risk).
Risk Group 4 (RG4)
Agents that are likely to cause serious or lethal human disease for which preventive or therapeutic interventions are not usually available (high individual risk and high community risk) Tissue Culture/Cell Lines.
Risk Group 1/Biosafety Level 1 (BSL1)
The following are considered Risk Group 1 cell lines and can be handled using BSL1 containment:
-
Cell lines of non-primate origin, which do not harbor a primate virus nor are they contaminated with an infectious organism.
Risk Group 2/Biosafety Level 2 (BSL2)
The following are identified as Risk Group 2 and must be handled at BSL2 containment:
-
Human and non-human primate cells including cell lines, tissue, blood and potentially infectious fluids.
- Cell lines exposed to or transformed by a primate oncogenic virus
- All clinical material (e.g., samples of human tissues and fluids obtained after surgical resection or autopsy for use in organ culture or establishment of primary cell cultures)
All unfixed human tissues and cells should be treated as infectious (the concept of “universal precautions”). All individuals who work in a laboratory containing these materials have the potential to be exposed to these materials. This requires these individuals must be included in the Bloodborne Pathogens program. These persons must be offered the hepatitis B vaccination and receive annual bloodborne pathogens training.
When cell cultures are known to contain an etiologic agent or an oncogenic virus, the cell line can be classified at the same level as that recommended for the agent or virus.
Chapter 5 – Routes of Transmission
The risk of exposure to biological agents in a research environment depends on several parameters (e.g., pathogenicity, virulence, infectious dose, communicability, subject’s susceptibility, route of transmission, etc.). The biosafety procedures used are designed to prevent exposures by containing the agents. To properly design the containment, it is important to recognize the potential routes of transmission for the given agent.
Skin and Mucous Membrane Contact
Decanting of liquids, pipetting, removal of screw caps, vortex mixing, streaking agar plates, and inoculation of animals may all result in the generation of infectious aerosols or droplets. Any direct contact between the infectious material and the subject’s skin, mucous membranes, or eyes may serve as a direct route of exposure.
Ingestion
Splashing of material into the mouth or indirect oral exposure through touching the mouth with contaminated hands can result in the ingestion of infectious material. Storage and/or consumption of food, drinks, or eating utensils in the lab can also lead to ingestion of infectious material. Mouth pipetting presents an extremely high risk of ingestion and is prohibited at MSU.
Percutaneous Inoculation
Use of syringes and needles are considered the greatest risk of exposure through inoculation. Accidental inoculation can also occur by way of cuts and scratches incurred from contaminated syringes used for animal inoculations, or from the animals themselves in the form of bites or scratches.
Inhalation of Aerosols
Many procedures have the potential for generation of aerosols, including sonication, centrifugation, “blowing out” of pipettes, heating inoculating loops, and changing litter from the cages of infected animals.
Chapter 6 - Biosafety Principles
Biosafety principles are used to provide safe methods for managing infectious agents in the laboratory environment where they are being handled or maintained. The purpose of biosafety principles is to reduce or eliminate exposure of laboratory workers, general public, and the outside environment to potentially biohazardous materials. The four biosafety principles are engineering controls, standard operating procedures, personal protective equipment, and administrative controls.
Engineering Controls
Engineering controls includes facility design, biological safety cabinets (BSCs), enclosed containers, safety centrifuge cups, and other controls designed to minimize exposure to biological agents.
Primary Engineering Controls
Primary engineering controls are designed to protect lab personnel and the immediate environment from exposure to infectious agents. They are the most effective at minimizing exposure when workers are trained in their proper use, and the equipment is regularly inspected and maintained. BSCs are the most important primary engineering control for protection of personnel and laboratory and may also provide protection for scientific samples and reagents.
Secondary Engineering Controls
Protecting the laboratory’s external environment from exposure to biohazardous materials is accomplished by a combination of biosafety principles and facility design. Facility airflow, access control, sinks for hand washing, self- closing doors, and heat-resistant and non-porous lab benches make up some of the most important examples of secondary engineering controls.
Standard Operating Procedures (SOPs)
This document, the Biosafety in Microbiological and Biomedical Laboratories (BMBL), and the NIH Guidelines provide general requirements for working with biological agents at MSU. However, because these documents cover relatively general topics, individual laboratories are required to develop laboratory-specific SOPs which cover their own specific biosafety concerns and laboratory procedures.
For example, laboratory-specific SOPs should address safe manipulation of specific organisms, specific exposure control methods, specific decontamination, and waste-handling requirements. The laboratory-specific SOPs do not need to duplicate the more general SOPs contained in this manual or the CDC/NIH documents but should serve as supplements.
Personal Protective Equipment (PPE)
Personal protective equipment (PPE) includes safety eyewear, disposable gloves, , and lab coats. PPE should be complemented by wearing full-length trousers and closed-toe shoes (no sandals) in the laboratory setting. Additional PPE such as face shields, or appropriate respiratory protection may be needed based on risk assessment. Proper clothing and PPE supplement the containment measures provided by laboratory practices and safety equipment.
PPE is designed to protect laboratory workers from serious exposure to biohazardous materials and should be used in conjunction with appropriate engineering and administrative controls. At a minimum, staff must use lab coats, safety glasses, and disposable gloves whenever working in the laboratory.
Administrative Controls
Administrative controls are policies and procedures designed to assist with the safe handling of potentially biohazardous materials. They include training, medical surveillance, vaccinations, and access control.
Chapter 7 - Biosafety Levels
Four Biosafety Levels (BSL-1,2,3,4) represent combinations of laboratory practices, techniques, safety equipment, and laboratory facilities. Each combination is specifically appropriate for the operations performed and the documented or suspected routes of transmission of the biohazardous material, as well as for the laboratory function or activity. The recommended biosafety level for an organism represents the conditions under which the agent can be ordinarily handled safely.
NIH’s Guidelines for Research Involving Recombinant or Synthetic Nucleic Acid Molecules classifies “human etiologic agents” according to their relative pathogenicity, i.e., the ability to cause disease. Agents are categorized into four risk groups (RG), which are outlined in Chapter 4.
In general, the biosafety level used matches the highest RG classification of the organisms involved. For example, work with vaccinia virus, a Risk Group 2 (RG2) agent, should be conducted at BSL-2 or higher; simultaneous work with E. coli (RG1), Epstein-Barr virus (RG2), and Mycobacterium tuberculosis (RG3) should be conducted at BSL-3.
Descriptions of biosafety levels, as well as assigned biosafety levels for specific organisms, are contained in the CDC/NIH document, Biosafety in Microbiological and Biomedical Laboratories (BMBL). The BMBL outlines four biosafety levels, summarized in the table below. Biosafety Level 2 guidelines are outlined in Appendix D.
|
BSL
|
Agents
|
Special Practices
|
Primary Barrier and Personal Protective Equipment
|
Facilites
(Secondary Barriers) |
|---|---|---|---|---|
|
1
|
Well-characterized agents not known to consistently cause disease in immunocompetent
adult humans and present minimal potential hazard to laboratory personnel and the
environment
|
Standard microbiological practices
|
No primary barriers required; protective laboratory clothing; protective face, eyewear,
as needed
|
Laboratory doors; sink for handwashing; laboratory bench; windows fitted with screens;
lighting adequate for all activites
|
|
2
|
Agents associated with human disease and pose moderate hazards to personnel and the
environment
|
Limited access; occupational medical services including medical evaluation, suveillance,
and treatment, as appropriate; all procedures that may generate an aerosol or splash
conducted in a BSC; decontamination process needed for laboratory equipment
|
BSCs or other primary containment device used for manipulations of agents that may
cause splashes or aerosols; protective laboratory clothing; other PPE. including respiratory
protection, as needed
|
Self-closing doors; sink located near exit; windows sealed or fitted with screens;
autoclave available
|
|
3
|
Indigenous or exotic agents; may cause serious or potentially lethal disease through
the inhalation route of exposure
|
Access limited to those with need to enter; viable material removed from laboratory
in primary and secondary containers; opened only in BSL-3 or ABSL-3 laboratories;
all procedures with infectious materials performed in a BSC
|
BSCs for all procedures with viable agents; solid front gowns, scrubs, or coveralls;
two pairs of gloves, when appropriate; protective eyewear, respiratory protection,
as needed
|
Physical separation from access corridors; access through two consecutive self-closing
doors; hands-free sink near exit; windows are sealed; ducted air ventilation system
with negative airflow into laboratory; autoclave available, preferably in laboratory
|
|
4
|
Dangerous and exotic agents that pose high individual risk of aerosol-transmitted
laboratory infections and life-threatening disease that are frequently fatal, for
which there are no vaccines or treatments; and related agents with unknown risk of
transmission
|
Clothing change before entry; daily inspections of essential containment and life
supprt systems; all wastes decontaminated prior to removal from laboratory; shower
or exit
|
BSCs for all procedures with viable agents; air-supplied, positive-pressure suit
|
Entry sequence; entry through airlock with airtight doors; c walls, floors, ceilings
form sealed internal shell; dedicated, non-recirculating ventilation system required;
double-door, pass-through autoclave required
|
Chapter 8 - Laboratory Biosafety Practices
Individuals working with infectious organisms or biohazardous materials must be aware of potential hazards and be trained and proficient in the practices and techniques required for handling such material safely. The PI is responsible for ensuring that laboratory personnel are properly trained; the PI may delegate the provision of training to the laboratory supervisor, but the responsibility remains with the PI.
Each laboratory must develop a lab-specific biosafety manual identifying specific hazards that may be encountered, along with specific practices and procedures that will minimize risk(s) to lab personnel. All lab members are required to read and follow the required practices, procedures, and be apprised of any special hazards. The PI or supervisor who is directing laboratory activities should be well-trained, experienced, and knowledgeable in the appropriate laboratory techniques, safety procedures, and hazards associated with the handling of infectious organisms and/or biohazardous material.
When standard laboratory practices are insufficient for the control of specific hazard(s) from an infectious organism, biohazardous material, or procedure, the PI will select additional safety measures to prevent exposure, and thereby ensure the safety of his/her lab personnel. These practices must also be supplemented by appropriate engineering controls, administrative controls, PPE, and SOPs.
The PI has ultimate responsibility for ensuring that persons working in the laboratory are adequately trained and that they follow the appropriate safety measures.
Basic Laboratory Practices
Prudent practices and good techniques are of primary importance in laboratory safety. Both are based on sound technical knowledge, experience, and an attitude of courtesy and consideration for others.
Techniques and practices are described in detail as “Standard Microbiological Practices” in the CDC/NIH’s Biosafety in Microbiological and Biomedical Laboratories and the NIH Guidelines.,
Standard microbial practices include the following:
-
Do not eat, drink, smoke, or apply cosmetics in the laboratory.
-
Wash hands after work is complete, after gloves are removed, or if there is a glove compromise (i.e., glove tear).
-
WearPPE at all times when working: lab coat, disposable gloves (e.g., nitrile or latex), and safety glasses.
-
Donot mouth pipette.
- Decontaminate work surfaces before and after work, and immediately after spills.
- Perform all manipulations that have the ability to create aerosols in a biological safety cabinet (BSC), or other containment device.
- Reduce the use of needles and other sharps when possible.
Laboratory Housekeeping and Personal Hygiene
Personal safety is greatly enhanced by keeping the workspace neat, clean, and orderly. Injuries and exposures are more likely to occur in poorly maintained, disorderly areas. All materials must be properly labeled, and waste discarded.
The following guidelines will be observed in the laboratory:
-
Routine housekeeping and regular disinfection of lab equipment are necessary to ensure work areas are free of sources of contamination and hazards.
- Laboratory personnel are responsible for cleaning laboratory benches and equipment.
- Access to exits, sinks, eyewashes, emergency showers, and fire extinguishers must not be blocked.
- The workplace should be free of physical hazards.
- Equipment should be properly grounded. Overloaded electrical circuits and the creation of electrical hazards in wet areas must be avoided.
- Surfaces must be clean and free of infrequently used reagents, glassware, and equipment.
- Eliminate trip hazards (e.g., items on floors, under benches, or in corners).
- All compressed gas cylinders must be properly secured.
Proper hand washing immediately after de-gloving ensures that potential contamination of the hand is removed before being spread.
The laboratory is an inappropriate place to perform personal cosmetic tasks, such as applying makeup, cleaning or trimming fingernails, or brushing hair. These activities provide a potential for exposure and may contribute to contamination of the laboratory environment.
Universal Precautions
The principal of universal precaution is defined by the Bloodborne Pathogens (BBP) Standard. This practice should be adopted by all laboratory personnel. Universal precautions require that all human blood, tissues, and other bodily fluids be handled as though they are infectious. Adopting and applying universal precautions to all laboratory activities creates an awareness of potential risks and adds another level of vigilance.
Biological Hazard Information
Laboratory workers must be knowledgeable about the hazards associated with the infectious organisms or biohazardous materials in their labs. Detailed hazard information, including Pathogen Safety Data Sheets, is available to all laboratory workers.
Storage and Labeling of Biological Agents
Biological agents must be stored using leak proof and sealed primary containers. Containers must be clearly labeled with the identity of the biohazardous material(s). At a minimum, secondary containers must include the universal biohazard symbol (identity of contents is also desirable).
Freezers, refrigerators, and other storage areas must be labeled with the biohazard symbol. Waste and contaminated equipment or other objects to be decontaminated must also be labeled with the biohazard symbol.
The OSHA Bloodborne Pathogen Standard specifically requires that all containers of human blood or other potentially infectious material (OPIM), including contaminated waste, refrigerators, freezers, and other storage or transport vessels be labeled with the universal biohazard symbol.

Biohazard Labels and Signs
Each laboratory must have a sign at the entrance that provides safety information to visitors and service personnel. Room signs must contain designations for all laboratory hazards in use within the laboratory (carcinogens, acutely toxic agents, reproductive hazards, biohazards, radioactive materials, lasers, and magnetic fields). Safety and Risk Management will prepare the signs for each door in accordance with the requirements of NFPA 704.
Biohazard signs will be posted at the following:
- Entrances to laboratories and animal rooms that use agents classified as BSL-1 or BSL-2.
- Cages or animal rooms used for housing animals infected with BSL-1 or BSL-2 agents.
Certain other areas and equipment within a laboratory may also require signs. Refrigerators, freezers, cabinets, and other storage facilities require the biohazard symbol whenever they are used to store infectious organisms or biohazardous material; human blood or blood products; unfixed tissues; cell or organ cultures; body fluids; or excreta.
Large pieces of equipment for handling such materials (e.g., centrifuges, biological safety cabinets) must be similarly labeled.
Microbial Agents
-
The CDC/NIH’s Biosafety in Microbiological and Biomedical Laboratories (BMBL) has descriptions of biosafety levels and recommended biosafety practices for specific biological agents.
-
The Public Health Agency of Canada maintains Pathogen Safety Data Sheets for biological agents.
Toxins
Isolated biological toxins are chemical hazards, although many such toxins produce adverse effects at doses significantly below that of “traditional” laboratory chemicals. A safety data sheet (SDS) for a specific toxin should be obtained from the vendor upon receipt of the toxin. Use of biological toxins require IBC approval before work is initiated. The MSU Biological Toxin Policy has additional details on biological toxin use at MSU.
Security and Inventory of Biological Agents
Each PI must develop site-specific criteria that safeguard all biological materials, regardless of their risk group, from unauthorized removal. It is the PI’s responsibility to ensure that his/her laboratory implements sufficient security measures and procedures to prevent unauthorized access to biological agents.
Each PI shall have an inventory of the biological materials stored in the laboratory.
Prevention of Aerosols and Droplets
Handling of liquids or dry powders often generates aerosols or droplets. High-energy procedures such as centrifuging, vortexing, and mixing, tend to produce aerosols that stay airborne for extended periods and are small enough to be inhaled; low-energy procedures, including opening containers and streaking plates, produce droplets that settle quickly on surfaces, skin, and mucous membranes.
Biological Safety Cabinets
The following guidelines are recommended when using biological safety cabinets (BSCs). Refer to Appendix B for differences between laboratory hoods.
-
The BSC must be certified when it is installed or after it is moved, and annually thereafter (for information on cabinet certification contact the BSO).
- The Magnehelic gauge should be checked regularly. This gauge will normally run at a relatively fixed value. The value is written on the certification sticker at the time of certification. When it deviates significantly, the cabinet must not be used until the cause of the deviation has been identified and fixed.
- Personnel must understand how the BSC works.
- If the BSC contains a UV light, personnel must be familiar with the safe and effective use of any UV lamps inside the BSC and use appropriate precautions to avoid UV-related injuries. The UV light is not considered a method of decontamination.
- The BSC’s protective airflow pattern should not be disrupted. Rapid arm movement, nearby workers, and open laboratory doors may disrupt the airflow pattern and reduce the cabinet’s effectiveness.
- The BSC must run for at least 5 minutes to allow for stabilization of airflow before any procedures are begun.
- The BSC must be left running whenever the cabinet is in use.
- Work and the necessary materials should be planned to minimize the need to exit and reenter the BSC.
- Accumulation of materials in the BSC should be minimized to reduce turbulence and ensure proper laminar airflow.
- Work surface must be disinfected after each use.
- Any piece of equipment (e.g., centrifuge, blender) capable of creating air turbulence should be placed in the back one-third of the BSC. All other work should be stopped while this equipment is operating.
- Open flames are not allowed inside the BSC because they create airflow turbulence which compromises sterility. Electric devices, such as loop sterilizers, are satisfactory alternatives to open flames.
- Flammables and other volatile chemicals are not permitted to be used in a BSC, with the exception of chemical disinfectants.
- A pan with disinfectant and/or a sharps container is placed inside the BSC for pipette/sharps disposal. Vertical pipette discard canisters on the floor outside the cabinet should be avoided.
- Contaminated and clean items should be segregated, and personnel should work from “clean to dirty.”
- A biohazardous waste collection bag is placed in the BSC to collect waste.
- Do not block air flow into the front and rear grilles.
- All spills in the cabinet must be cleaned immediately. Work must cease until spill is appropriately cleaned according to MSU spill procedures.
- When work is complete, all materials must be disinfected before being removed from the BSC, and all interior surfaces must be wiped with appropriate disinfectant.
- Gloves must be removed, after touching or handling contaminated materials or if compromised (torn or hole).
- Laboratory coats must be removed, and hands thoroughly washed before leaving laboratory.
Utilization of Pipettes
Pipettes are used for volumetric measurements and the transfer of fluids that may contain infectious, toxic, corrosive, or radioactive agents. Laboratory-associated infections have occurred from oral aspiration of infectious materials, mouth transfer via a contaminated finger, touching face (eyes, nose, etc.) and inhalation of aerosols. Exposure to aerosols may occur when liquid from a pipette is dropped onto the work surface; when liquids are mixed by pipetting (creation of bubbles); or when the last drop of liquid is blown out.
The following safe pipetting techniques will minimize the potential for exposure to hazardous materials:
-
Never mouth pipette. Always use a pipetting aid.
- Do not prepare infectious organisms or biohazardous materials by bubbling expiratory air through a liquid with a pipette.
- Do not forcibly expel infectious organisms or biohazardous material out of a pipette.
- When pipetting, avoid accidental release of infectious droplets.
- Do not discharge material from a pipette at a height above the receptacle. Whenever possible, allow the discharge to run down the container wall instead.
- Place contaminated, reusable pipettes horizontally in a pan containing enough liquid disinfectant to completely cover them.
- Discard contaminated, broken, or intact Pasteur pipettes and broken glass in a sharps container.
- Dispose of the sharps container properly when it has reached the full line marked on the container.
- Pans or sharps containers for contaminated pipettes should be placed inside the BSC.
- Proper procedures for disposal of plastic pipettes are presented in Chapter 14.
Utilization of Centrifugation
Hazards associated with centrifuging include mechanical failure and the creation of aerosols. To minimize the risk of mechanical failure, centrifuges must be cleaned regularly, maintained, and used according to the manufacturer’s instructions. Users must be trained on proper operating instructions that include safety precautions of the centrifuge unit.
Aerosols are created by activities such as filling centrifuge tubes, removing plugs or caps from tubes after centrifugation, removing supernatant, and re-suspending pellets. A significant aerosol hazard can also be created if a tube breaks during centrifugation.
To minimize the generation of aerosols when centrifuging biohazardous material, the following procedures are recommended:
-
Use sealed tubes and safety buckets that seal with O-rings. Before use, inspect tubes, O-rings, and buckets for cracks, chips, erosions, bits of broken glass, etc.
- Fill and open centrifuge tubes, rotors, and accessories in a BSC, if appropriate.
- Ensure tubes being used are rated for the centrifugation speeds required.
- Avoid overfilling centrifuge tubes to prevent closures from becoming wet. After tubes are filled and sealed, wipe them down with disinfectant.
- In the event of breakage during centrifugation, rotors/buckets should be opened inside of a BSC, and the centrifuge should be immediately decontaminated.
- Always balance buckets, tubes, and rotors properly before centrifugation.
- Avoid decanting or pouring off supernatant; use a pipette to remove the supernatant.
- Work in a BSC when re-suspending material. Use a swirling rotary motion rather than shaking. If shaking is necessary, wait a few minutes to permit the aerosol to settle before opening the tube.
- Small, low-speed centrifuges may be placed in a BSC during use
Utilization of Cryostats
Use of cryostats is very common in many research laboratories. These devices pose potential hazards associated with sharp cutting edges and cold environments and must be handled with extra care.
The following guidelines should be followed when using cryostats:
- Frozen sections of unfixed human tissue or animal tissue infected with an etiologic agent pose a risk because freezing tissue does not necessarily inactivate infectious agents. Use of freezing propellants under pressure is not recommended with frozen sections because they may cause spattering of droplets of potentially infectious material.
- Appropriate gloves should be worn during preparation of frozen sections.
- When working with human or infected animal tissue, consider the contents of the cryostat to be contaminated and decontaminate it frequently with 70% alcohol.
- Consider all tissue remnants potentially infectious; carefully remove such accumulations from the cryostat during decontamination.
- Handle microtome knives with extreme care. Stainless steel mesh gloves should be worn when changing knife blades.
- Staining solutions used on potentially infected frozen sections are treated as if they are contaminated and must be disposed of properly.
Utilization of Inoculating Loops
Flaming inoculating loops can result in spatter and the release of aerosols and droplets. Use of an electric micro incinerator is the preferred, safer alternative.
Use of Absorbent Materials
Work surfaces should be covered with absorbent paper or “diaper” sheets to collect splashes and drips, thereby minimizing the spread of contamination. The absorbent paper should be changed at the end of the laboratory procedure as part of the final cleanup, or at least daily.
Utilization of Miscellaneous Aerosol-Producing Devices and Activities
Use of any of the devices listed below results in considerable aerosol production. Blending, cell-disrupting, and grinding equipment should be used in a BSC when working with biohazardous materials.
Blenders
Safety blenders are designed to prevent leakage from the bottom of the blender jar. They provide a cooling jacket to avoid biological inactivation and can withstand sterilization by autoclaving.
-
Blenders must be tested to ensure they are leak proof prior to use with any biohazardous material. Blenders can be tested with sterile saline or dye solution.
-
The use of glass blender jars is not allowed because of the potential for breakage.
-
When opening blenders, be cognizant of potential contamination hazards in the form of droplets that might become airborne or fall on the surfaces; liquid residue on the cap; and possible expansion of the volume due to aeration.
-
Before opening the blender jar, allow the unit to rest for a minimum of five minutes to allow the aerosol to settle.
-
Effective control of contamination can be achieved by placing the blender in a tray lined with absorbent pads, and inside a BSC.
- The device should be decontaminated after use.
Lyophilizers
Depending on lyophilizer design, aerosol production may occur when material is loaded into or removed from the lyophilizer unit.
-
The vacuum pump exhaust must be filtered to remove any hazardous agents.
-
After lyophilization is complete, all potentially exposed surfaces of the unit must be disinfected with the appropriate disinfectant.
-
If the lyophilizer is equipped with a removable chamber, it should be closed off and moved to a BSC for unloading and decontamination.
-
Vapor traps must be used whenever possible.
- Use filter topped tubes when possible.
Sonicators
Sonication is the use of sound-wave energy for dispersion, disruption, or inactivation of biological materials. Sonicators generate sound waves at very high frequencies (~20,000 + Hz range), which is outside normal hearing range. Be aware of these hazards:
-
Noise: Although the 20,000-Hz frequency is outside normal hearing range, there are other sources of noise, such as vibration from any loose equipment or other items on the bench or the liquid itself. Contact Safety and Risk Management for a noise evaluation.
- Tubes must have lids closed when utilizing sonicating water bath.
- Sonicators that utilize a vibrating probe must be used inside of a BSC when using biological agents in a BSL2.
-
Aerosols: Aerosols present a more serious potential hazard and must be taken into consideration.
-
Observe all precautions listed above for blenders and lyophilizers.
Ampoules
Opening ampoules containing liquid or lyophilized culture material should be performed in a BSC to contain aerosols. Sealed-glass ampoules used to store biohazardous material in liquid nitrogen have exploded, causing eye injuries. The use of polypropylene tubes (cryovials) eliminates this hazard.
Polypropylene cryovials are available in dust-free or pre-sterilized forms; each tube is fitted with a polyethylene cap and a silicone washer. Heat-sealable polypropylene tubes are also available.
- To open a sealed-glass ampoule, nick the neck of the ampoule with a file, wrap it in disinfectant- soaked disposable towel, hold the ampoule upright, and snap it open at the nick.
- Reconstitute the contents of the ampoule by adding liquid slowly to avoid aerosolizing the dried material.
- Mix the contents without bubbling and withdraw it into a fresh container. Discard the disposable towel and the ampoule’s top and bottom as biohazardous waste.
Loop Sterilizers and Bunsen Burners
Sterilization of inoculating loops or needles in an open flame generates small-particle aerosols that may contain viable microorganisms.
- Alternatively, disposable plastic loops and needles may be used for culture work where electric incinerators or gas flames are unavailable.
- Gas burners may not be used in a BSC. These burners can produce turbulence that disturbs the cabinet’s protective airflow patterns. Gas leaks can concentrate flammable gas and cause explosions.
-
Electric sterilizers and micro incinerators must be used when working in a BSC.
Chapter 9 – Engineering Controls
Primary Engineering Controls
Biological Safety Cabinets (BSC)
BSCs constitute one of the most critical pieces of safety equipment in Biosafety Level (BSL) Containment laboratories. Designed to contain aerosols generated from biological material via laminar air flow and high efficiency particulate air (HEPA) filtration, BSCs differ from chemical and laminar flow hoods (clean hoods) in that they always offer personnel projection. They also provide some protection from contamination of the material being handled within the work environment.
There are three types of BSCs (Class I, II, and III); each offers different levels of protection. Open-fronted Class I and Class II BSCs are partial containment devices that provide a primary barrier; significant levels of protection of personnel and environment are obtained when complemented by good laboratory technique. The gas-tight Class III BSC, or glove box, provides the highest level of protection to personnel, environment, and product.
The Class I BSC is suitable for work where there is a need for protection from the biological material, but not for protection of the product. It protects personnel and environment from contaminants within the BSC but does not protect the work within the cabinet from “dirty” room air.The Class II BSC protects the material being manipulated inside the cabinet (e.g., cell cultures, microbiological stocks) from external contamination. It meets requirements to protect personnel, the environment, and the product. The two basic types of Class II BSCs are Type A and Type B. The major differences between Types A and B may be found in the percent of air that is exhausted or recirculated, coupled with the way exhaust air is removed from the work area.
The gas-tight Class III BSC, or glove box, provides the highest level of protection to personnel, environment, and product. It is the only unit that provides a total physical barrier between the product and personnel. It is used with high-risk biological agents and when absolute containment of highly infectious or hazardous material is required.
It is important to note that laminar flow hoods (clean hoods) or chemical fume hoods must not be utilized for work with biological agents. Laminar flow hoods provide product protection by ensuring that the product is exposed only to HEPA-filtered air. They do not provide protection to personnel or the laboratory environment. Chemical fume hoods only provide personal protection by directional airflow into the fume hoods, which prevents chemical fumes from exiting out of the hood. Instead, the air is exhausted to the outside, generally above the roof of the building. They do not provide HEPA filtered air at all, and are therefore unsuitable to protect personnel, product, or environment.
BSC Owners Responsibilities and BSC Maintenance
Proper operation and maintenance of a BSC requires knowledge of how the system operates, as well as training and experience in effective techniques for working within the BSC without compromising its functions. Additional details concerning the design and use of BSCs are provided in Appendix C.
Two specialized forms of quality control are strongly recommended for all BSCs:
-
At least daily, or each time the cabinet is operated, the user should observe the Magnehelic gauge and note its relative position. Magnehelic gauges measure the pressure drop across the outlet HEPA filter and are important indicators of filter integrity and loading. The gauge will typically indicate the same measurement over a long period of time. A significant change in the reading over a short period of time may indicate clogging or a leaking filter. In such cases, the hood should not be used until the problem is identified and resolved. If the BSC does not have a Magnehelic gauge, users must understand the operation of the airflow monitor, controls, and alarm settings.
-
Annually, the cabinet must be certified by licensed technician. The certification process ensures that the BSC is meeting its operating specifications and providing maximum protection. In addition, technicians can provide services and preventive maintenance for BSCs and can often forecast expensive requirements like HEPA filter replacements, thereby enabling PIs to budget for the event.
- BSCs must be recertified after relocation.
-
Annual BSC recertification must be completed before the current certification expires. If the certification lapses, the BSC may not be used for BSL-2 or higher procedures until it is recertified. Laboratory personnel should report lapsed BSC recertification to the Biosafety Officer immediately. The Biosafety Officer will inform the PI and lab workers not to use the BSC, post a “DO NOT USE” sign on it, and will arrange for a technician to recertify the BSC as soon as possible. The certificate expires on the last day of the month in which the certification was performed, one year later (for example, a certificate issued on June 2, 2022 will expire on June 30, 2023).
PIs are responsible for ensuring the proper maintenance and care of BSCs. Contact the BSO for more information.
Secondary Engineering Controls
Facility Design
The design of a laboratory facility is important in providing a barrier to protect both the personnel working within, as well as those outside of the laboratory. It should also protect the surrounding community and environment from accidental release of infectious agents in the lab. Facility design must be commensurate with the laboratory's function, particularly the BSL required for use and/or storage of the biological agents therein.
The recommended secondary barrier(s) will depend on the risk of transmission of specific agents. For example, the exposure risks for most laboratory work in BSL-1 and BSL-2 facilities will be direct contact with the agents or inadvertent contact exposures through contaminated work environments. Secondary barriers in these laboratories may include separation of the laboratory work area from public access; availability of decontamination equipment (e.g., autoclave*); and sinks for handwashing. In BSL-3 facilities, additional safeguards, such as directional airflow, airlock-controlled entry and exit, and a shower unit for personnel.
As the risk for aerosol transmission increases, higher levels of primary containment and multiple secondary barriers may become necessary to prevent infectious agents from escaping into the environment. Such design features may include specialized ventilation systems to ensure directional airflow; air treatment systems to decontaminate or remove agents from exhaust air; controlled access zones; an airlock at the laboratory entrance; and separate buildings or modules for physical isolation of the laboratory itself.
*Note: Autoclaves used to sterilize biohazardous materials must be validated monthly; typically, a sporulation test is used. Validation records are essential. Refer to the MSU Autoclave Quality Assurance Program for additional details. Biohazardous materials can also be disposed of in a red bag as medical waste (without autoclaving) and will be picked up by SRM. A nominal fee is charged for this service.
Chapter 10 - Personal Protective Equipment (PPE)
A Primary Barrier
Personal protective equipment (PPE) must be provided without cost to personnel. Although not a substitute for the use of BSCs and good laboratory practices, PPE is considered a primary barrier to infectious agents. Proper use of PPE significantly reduces the likelihood of infection; however, it is the least-desirable exposure control method because its failure will result in direct exposure to the agent.
All PPE must be removed and left in the lab prior to exiting for non-laboratory areas (e.g., hallway)
PPE is most effective when used to supplement primary control methods such as BSCs, safety centrifuge cups, and other containment devices. Appropriate PPE and clothing also protect experiments from contamination.
Face Protection
At minimum, safety glasses are required when working in a MSU laboratory. Eye protection should conform to the Standard for Occupational and Educational Eye and Face Protection, Z87.1, established by the American National Standards Institute (ANSI). Goggles, masks, chin-length face shields, or other splatter guards are required for anticipated splashes, sprays, or splatters of infectious or other hazardous materials to the face. Wearing contact lenses is inappropriate in the laboratory setting because contacts may trap infectious agents against the surface of the eye and prevent effective purging by eye washing.
Laboratory Clothing
Laboratory coats, smocks, scrub suits, and gowns are considered proper laboratory clothing.
- Long-sleeved garments are used to minimize the contamination of skin or street clothes and to reduce shedding of microorganisms from the arms
- In circumstances where it is anticipated that splashes may occur, the garment must be resistant to liquid penetration to protect clothing from to protect clothing from contamination.
- If the garment is not disposable, it must be capable of withstanding sterilization (generally, autoclaving), should it become contaminated.
- Protective clothing must be removed and left in the laboratory before leaving for non-laboratory areas.
- Disposable clothing should be available for visitors, as well as maintenance and service workers. All protective clothing should be discarded in the laboratory, disinfected, and/or laundered by the facility.
- Personnel must not launder laboratory clothing at home. If facilities/labs wish to have laboratory clothing laundered at a commercial laundry facility the laboratory clothing must be autoclaved prior to laundering.
Gloves
Gloves must be selected according to the hazards involved and the activities to be conducted.
- Gloves must be worn when working in a MSU laboratory.
- Always wash hands with soap and water after removing gloves.
- Gloves must be removed and disposed of in biohazardous waste when:
- contaminated
- compromised/torn,
- work with infectious materials is completed
- Temperature-resistant gloves must be worn when handling hot materials, dry ice, or materials being removed from cryogenic storage devices.
- When working with hazardous materials, the glove should overlap the lower sleeve and cuff of the laboratory garment. A long-sleeved glove or disposable arm-shield may be worn for further protection of the garment.
- In some instances, double gloving may be appropriate. If a spill occurs, hands will be protected after the contaminated outer gloves are removed.
- Disposable gloves must not be washed or reused.
- Protection from contact wth toxic or corrosive chemicals may also be required. For assistance in glove selection, call SRM.
Respirators
Respirators are selected based on the hazard involved and the protection factor required. Certain laboratory and clinical situations require respiratory protection to prevent inhalation of infectious agents.
Regulations, as well as good safety practices, require that personnel be medically evaluated, specifically trained, and fit- tested prior to wearing respiratory protective equipment.
Contact SRM if respiratory protective equipment is required or if there are questions about the Respiratory Protection Program.
Footwear and Miscellaneous Clothing Guidelines
Open-toed shoes or sandals are not allowed in the lab. In addition, wearing shorts or other clothing that exposes the lower legs is not allowed.
Chapter 11 - Training for Laboratory Personnel
Training is a critical component of any biological safety program. Training is intended to provide the understanding, technical knowledge, and tools that the trainee can use to improve his/her daily laboratory safety practices.
At a minimum, all MSU personnel working with biological materials must have training in the following areas prior to the start of their experiments:
-
Knowledge of this biosafety manual
-
Experimental procedures to be used
-
Decontamination and spill clean-up procedures
- Safe handling methods for any infectious agents and/or recombinant/synthetic nucleic acid molecules (e.g., rDNA)
-
Proper methods for transporting infectious agents and other biohazardous materials
-
Bloodborne Pathogens Standard (if they work with human blood or blood products, unfixed tissue, body fluids, organ, or primary tissue and/or samples contaminated with bloodborne pathogens)
-
Other specialized biosafety training as deemed appropriate by the IBC or the BSO.
The PI is responsible for ensuring that his/her employees receive proper training in the biohazards and controls specific to his/her laboratory and the safe conduct of the experimental procedures to be used. The Biosafety Program provides different types of training associated with the various laboratory programs at MSU.
Mandated General Biosafety Training
Training is required by law and MSU IBC Policy. Specific training requirements are outlined on the MSU Biosafety Training website. Training is offered in person (see schedule on this page) or online via CITI (see instructions on this page).
Training is required for all laboratory workers (faculty, staff, students, and visiting scientists) at MSU. The exact training required for a specific individual will depend on the hazards to which the worker is exposed.
New employees, faculty, and staff must attend this training program immediately following their hiring or as soon as practical and before beginning laboratory work. Attendance at new employee orientation does not fulfill this requirement. Training includes, but is not limited to, laboratory safety practices, biosafety, bloodborne pathogens, and hazardous waste operations.
Laboratory safety training satisfies the basic competency regulatory requirements for those working in labs. It does not satisfy the need for department-specific training, select agents, Biosafety Level 3 work, or other specialized training.
Mandated Specific Training
Mandated specific training is also required by law and/or policy. In some cases, it is administered and tracked by the BSO, who maintains the training records. Examples of mandated specific training include agent specific trainings, or other specific training required by the IBC/Federal regulations. Individuals working in laboratories classified as BSL-3, or who are potentially exposed to specific zoonotic diseases, must also undergo specific training.
Training laboratory personnel in the unique hazards, equipment, and procedures for a given laboratory is the responsibility of the PI or laboratory manager to administer, document, and track. This training is mandated and must be provided to all laboratory personnel on a periodic basis. Documentation is also required and must include at least the date and duration of training, name and position of the trainer, topics covered, and names of the trainees.
Packaging and Shipping of Infectious Agents Training
Personnel who package and ship infectious agents and diagnostic specimens such as microorganisms, blood samples, and clinical samples for pathological testing are required by federal and international regulations to receive training every two years. This training is offered on demand through CITI, or periodically through coordination with the BSO.
Laboratory-Specific Training
Individual laboratories are required to develop training tailored for specific agents and/or procedures that personnel will perform in that laboratory. This training should be specific to the hazards in the laboratory and to each person’s laboratory duties. All laboratory personnel must understand the hazards associated with the agent and laboratory operations, how to prevent exposures to biological and chemical agents (see Chemical Hygiene Plans) and be trained in the laboratory standard operating procedures (SOPs). Laboratory-specific training supplements general biosafety training.
Training Records
Each laboratory must maintain training records, which should include the names of lab personnel, and their completion dates for safety training as well as lab- and/or agent-specific training provided by the PI or lab supervisor. All records must be updated and maintained by the PI or supervisor. Ongoing training is required as new hazards and procedures are introduced into the laboratory. The occurrence of spills, spread of contamination, near misses, etc., may also indicate the need for refresher training.
Other Safety Training
Personnel who utilize hazardous chemicals, radioisotopes, or x-ray-generating devices must attend additional safety training geared toward these specific hazards.
Updating Training
All laboratory workers must update their training per the IBC requirements.
Chapter 12 - Decontamination and Sterilization
Decontamination is a process or treatment that renders a device, instrument, or work surface safe to handle. A decontamination procedure can range from sterilization by autoclave or hydrogen peroxide vapor (i.e., VHP) to simple cleaning with soap and water. Sterilization, disinfection, and antisepsis are all forms of decontamination.
Sterilization is the use of a physical or chemical procedure to destroy all microbial life, including highly resistant spores.
Disinfection eliminates virtually all pathogenic, non-spore-forming microorganisms but not necessarily all microbial forms on inanimate objects (work surfaces, equipment, etc.). Effectiveness is influenced by the kinds and numbers of organisms, the amount of organic matter, the object to be disinfected, and chemical exposure time, temperature, and concentration.
Antisepsis is the application of a liquid antimicrobial chemical to skin or living tissue to inhibit or destroy microorganisms. It includes using germicidal solutions to swab an injection site on a person or animal, as well as for handwashing. Although some chemicals may be utilized as either a disinfectant or an antiseptic, adequacy for one application does not guarantee adequacy for another. Manufacturers’ recommendations for appropriate use of germicides should always be followed.
General Procedures
Decontamination of cultures and objects contaminated by biological agents is routinely performed in laboratories and is a vital component of microbiological safety practice. It not only serves to protect laboratory personnel (as well as any bystanders) from infection, but also prevents the release of infectious organisms to the outside environment. Decontamination of media, work surfaces, and equipment is also necessary to prevent contamination of experimentally cultured organisms.
Infectious waste materials such as liquid and solid will be handled, treated and disposed of according to hazardous waste policies and procedures. Liquid wastes such as bacterial or viral culture media from BSL-2 labs will be treated with appropriate disinfectant prior to sink disposal. Solid waste from BSL-2 laboratories will be segregated and placed in biohazard containers lined with biohazardous waste bags and disposed as biological waste.
-
Autoclaving is the preferred method for treating biological waste.
- A disinfectant must appropriate for the organism in use.
- All liquid biological cultures must be inactivated with appropriate disinfectant for the appropriate contact time.
- All solid biological waste must be disposed of in the biohazard waste.
Methods of Decontamination
The three main categories of physical and chemical decontamination are heat, liquid disinfection, and vapors /gases.
- Heat: Wet heat is the most dependable method of sterilization. Autoclaving (saturated steam under pressure of approximately 15 psi with a chamber temperature of at least 121° C/250° F for a prescribed time) is the best method of rapidly achieving destruction of all forms of microbial
- Sterilization requires that materials come in direct contact with the steam and heat. Indicators of proper autoclave operation (e.g., autoclave tape or autoclave-sensitive labels) must be used with each load to visually confirm successful processing.
- Use of autoclave tape alone is not an adequate monitor of autoclave performance. As mentioned previously, the Autoclave Quality Assurance Program requires the use of a well-documented, monthly sporulation test to confirm sterilization.
- Liquid disinfection: A liquid disinfectant (e.g., 1:10 solution of household bleach yielding a final hypochlorite concentration of 5%) is used to wipe or soak potentially contaminated materials to kill all pathogenic agents present. Each specific disinfectant requires its own specific contact time.
- Gas and vapor: Potentially contaminated articles are exposed to a sterilizing gas (e.g., ethylene oxide, or ETO) or vapors from a chemical (e.g., formaldehyde). Because of the hazardous nature of the gases and vapors used, this requires specially designed equipment and facilities.
Autoclaving
Autoclaving uses saturated steam under pressure to achieve a high temperature in the autoclave. Autoclaving can be used to destroy vegetative bacteria, bacterial spores, and viruses. When decontaminating biohazardous waste, it is recommended that the temperature in the waste reach a minimum of 121o C. The total processing time required to meet these conditions depends on several loading factors (see below); however, it is recommended that a minimum autoclave cycle of one hour be used when decontaminating waste.
When using an autoclave, the following guidelines should be taken into consideration:
- Biohazardous materials should not be placed in autoclaves overnight in anticipation of autoclaving the next
- Autoclaves must not be operated by untrained
- Precautions must be taken to prevent accidental removal of material from an autoclave before it has been sterilized.
- Dry hypochlorite, or any other strong oxidizing material, must not be autoclaved with organic materials such as paper, cloth, or oil.
Temperature: an autoclave uses steam under a pressure of approximately 15 psi to achieve a chamber temperature of at least 121o C. Although the autoclave chamber may reach 121o C, this does not necessarily mean that the interior of the load will reach this temperature.
Time: a minimum autoclave cycle time of 20 minutes at a chamber temperature of 121o C (time does not begin as soon as the autoclave cycle is initiated) is commonly recommended for sterilization of clean items. However, the total processing time required to achieve decontamination depends on several loading factors, including the load container (heat transfer properties); the amount of water added to the load; and the weight of the load. For increased loads, an increased cycle time will be required to ensure effective decontamination.
Contact: steam saturation is essential for maximum heat transfer. Steam must contact all areas of the load. Autoclave bags and other containers should be left partially open (or otherwise permit entry of steam) to ensure adequate contact. Studies have shown that adding water to the interior of the bag improves the time-temperature profile of the autoclave cycle, thereby increasing the autoclave’s sterilization efficiency.
Dry Heat
Requiring higher temperature and longer contact time, dry heat is less effective than moist heat (autoclaving). Nevertheless, dry heat is preferable to moist heat for decontamination of anhydrous materials and closed containers because the moisture component of the steam used in an autoclave will not effectively penetrate anhydrous materials and closed containers.
A temperature of 160o-180o C/320 o-356 o F for three to four hours is recommended for decontamination of waste using a dry heat oven.
Chemical Disinfection
Disinfection is the decontamination of work surfaces, equipment, BSCs, and other inanimate objects using antimicrobial agents. Several chemical agents are used as disinfectants.
Laboratory workers should remember that there are hazards associated with all chemical disinfectants.
- Inhalation and skin contact should be minimized, and eye contact avoided; PPE is essential.
- Appropriate gloves and safety eyewear should always be worn when handling these chemicals
Chapter 13 - Biohazardous Spill Response
Preparing for Biohazardous Spill Cleanup
Spill Cleanup Materials
Each laboratory must have a spill kit available to respond to the largest spill anticipated for that area. At a minimum, the following spill cleanup materials should be available in the laboratory:
-
Disposable gloves (e.g., nitrile)
-
Safety glasses
-
Lab coat or smock to protect clothing and body
-
Absorbent materials (e.g., Pig Pad, paper towels)
-
Disinfectant appropriate for the agents used in the laboratory
-
Forceps or other devices to pick up contaminated material (especially sharps)
-
Autoclavable biohazard bags
Note: The chemical spill kits distributed by SRM to MSU laboratories may not be adequate for the response to a biological spill.
Biohazardous Spill Cleanup Risk Assessment
Several factors must be considered when assessing the risk that a spill presents:
-
Volume and concentration of the spilled material
-
The infectious dose of the spilled material and routes of exposure
-
Location of the spill
-
Degree of aerosolization of the agent resulting from the spill
-
Susceptibility of the spilled material to disinfection
-
Nature of the affected surface(s)
As with any spill scenario (biological, chemical, or radiological), the safety of personnel is the most important consideration. Cleanup is to begin only after it is determined that the personnel who will clean up the spill have appropriate knowledge, training, and equipment.
Biohazardous Spill Cleanup Procedures
All spills involving recombinant and/or synthetic nucleic acid molecules (including biological agents that have been genetically altered) must be reported to the Biosafety Officer.
The following are general biohazardous spill cleanup procedures that are appropriate for most spill scenarios; however, the appropriate response to any spill is based on an assessment of the risk associated with that particular situation.
Call the BSO with any questions or concerning spills.
Biohazardous Spills Inside Biosafety Cabinets (BSCs)
-
Wear a lab coat, safety glasses, and gloves during cleanup.
-
Allow the BSC to run continually during cleanup.
-
Surround the affected spill area with absorbent material to prevent spread of the spill.
-
Apply disinfectant appropriate for the biological agent and allow a minimum of 20 minutes contact time (or as directed by manufacturer’s instructions). Alcohol or other flammable liquids are not recommended.
-
Wipe up the spill with a disposable cloth or a towel soaked with disinfectant.
-
Wipe the BSC’s walls and work surface, as well as any equipment in the cabinet, with a disinfectant- soaked cloth.
-
Place contaminated items in an appropriate container (biohazard waste bag, sharps container, or autoclavable pan with lid for reusable items) for autoclaving.
-
Remove protective clothing and place in a biohazard waste bag for autoclaving.
-
Thoroughly wash hands and forearms with soap and water.
-
Allow BSC to run for a minimum of 10 minutes before resuming work in the cabinet or shutting off the cabinet.
Biohazardous Spills in the Laboratory, Outside the BSC
- Alert others in the area.
- Remove any contaminated clothing and place in a biohazard waste bag for autoclaving
- Wash all areas affected by skin contact with soap and water.
- Wear a long-sleeved gown or lab coat, shoe covers, safety glasses, and gloves.
- Place absorbent material over the spill
- Pour disinfectant around the spill, working from the outside of the spill to the center.
- Disinfect all items in the spill area (e.g., counter, cabinet, equipment).
- Allow a minimum of 20 minutes contact time (or as directed by manufacturer’s directions) with the disinfectant.
- Place contaminated items in an appropriate container for autoclaving (biohazard waste
bag, sharps container, or autoclavable pan with lid for reusable items).
Use tongs/forceps or plastic scoops to pick up sharps. - Remove protective clothing and place in a biohazard waste bag for autoclaving.
- Thoroughly wash hands, forearms, first and then other potentially contaminated body partswith soap and water. It is recommended that cleanup personnel shower as soon as possible.
Biohazardous Spills Inside a Centrifuge
-
Clear the area of all personnel and allow aerosols to settle (usually a minimum of 30 minutes) before re-entering the area.
- Wear a lab coat, safety glasses, and gloves during cleanup.
-
Transfer the rotor and buckets to a BSC for cleanup. Do not open rotor or buckets outside of the BSC.
-
Using an appropriate disinfectant, thoroughly disinfect the inside of the centrifuge, the rotor, and buckets.
-
Discard cleanup materials and protective clothing as biohazardous waste.
-
Thoroughly wash hands, forearms, and other parts of the body with soap and water.
Biohazardous Spills Outside the Laboratory DuringTransport
All biological agents are to be transported from the laboratory must follow MSU’s Transporting Biological Agents policy. In the event a transport container drops, and its contents are spilled, the following procedures should be followed:
-
Immediately clear the area of all personnel and secure the area.
-
Cleanup should be initiated as soon as possible to prevent spread of aerosol.
-
Attempt cleanup only if appropriate cleanup materials and protective clothing are available.
-
Notify the BSO
When responding to a spill, the following rules should be followed:
-
Tend the injured: Ensure receipt of immediate medical care and do not attempt to move the injured individual(s) unless ambient conditions become life-threatening. Individuals splashed, sprayed with, or otherwise exposed to human blood or other body fluids or tissues during a spill will need to remove contaminated clothing and utilize basic first aid
-
Wash any wounds immediately with soap and water.
-
Await assistance: Unless laboratory personnel are trained and properly supplied with PPE, do not attempt to clean up the spill. Personnel should immediately call the Biosafety Officer
-
Isolate the spill: Evacuate the immediate spill area or the entire room in the case of an aerosolizing (splashing or spraying) spill or a spill of volatile material. Prevent others from entering the spill area with barricades or, if necessary, a sentry.
-
Remove contaminated PPE and don new PPE prior to cleaning spill.
-
Contain the spill: Place absorbent material around, on, or in the flow path of the spilled material only if it can be done safely.
-
Provide information: Provide the information requested by the Biosafety Officer or SRM personnel and await arrival of the emergency provider.
-
Clean up: Clean up should take place only if laboratory personnel are trained, properly supplied with PPE, and otherwise able to clean up and disinfect the spill safely.
Chapter 14 - Biohazardous Waste Disposal
All biohazardous waste must be properly treated prior to disposal. The following types of waste are identified and defined as biohazardous waste:
-
Biological agents listed in this plan
-
Human liquid blood, blood products and body fluids
-
Human tissues and anatomical remains
-
Materials contaminated or potentially contaminated with biological agents during the manipulation or clean-up of material generated during research and/or teaching activities
-
Animal carcasses, body parts, blood, fluids, and bedding from animals infected with RG2 or RG3 biological agents
- Animal carcasses, body parts, blood, fluids, and bedding from animals infected with genetically modified biological agents
-
Animal carcasses, body parts, blood, fluids, and bedding from animals known or suspected to be reservoirs for zoonotic agents (e.g., NHP materials)
Labeling Biohazardous Waste
All biohazardous waste must be labeled with the universal biohazard symbol, laboratory room number, PI, and list the biohazardous agents in the waste.
Handling and Disposal of Biohazardous Waste
Sharps
Sharps include all needles, lancets, scalpels, and other similar medical instruments (contaminated or not), as well as contaminated Pasteur pipettes and non-broken or broken glass, and other instruments or materials that can cut or puncture personnel.
- Sharps must be collected in closable, puncture resistant, leakproof on sides and bottom, and labeled with the biohazard symbol.
- Sharps containers must be designed so that sharps can be safely introduced into the container but not easily retrieved.
- When the sharps container is filled to the marked line on the exterior of the container, staff must seal the container and request a waste pickup through SRM.
Solid Biohazardous Waste
Solid biohazardous waste includes disposable PPE (e.g., gloves, shoe covers), microbial agents, disposable plasticware, disposable pipettes, and contaminated material (e.g., paper towels, Kimwipes). These materials are collected in orange or clear biohazard bags that are double-lined, loosely closed, labeled, and autoclaved. After autoclaving, waste is placed into black trash bags and disposed of in the dumpster.
Liquid Biohazardous Waste
Liquid biohazardous waste includes all blood and liquid waste from humans or animals, and all other contaminated liquid waste (e.g., microbial cultures). Collect liquid waste in sealable, rigid, leak-proof containers labeled with the universal biohazard symbol.
- Human and animal blood and body fluids can be disposed of by chemically disinfecting the liquid with bleach or other approved chemical, for the appropriate contact time, and then flushing directly to the sanitary sewer
- All other liquid waste must be autoclaved or treated with a disinfectant prior to disposal.
- Liquid waste treated with bleach or MicroChem can be disposed of by flushing directly to the sanitary sewer after sufficient contact time. Liquid waste treated with other chemical disinfectants must be disposed of as hazardous chemical waste through SRM.
Animal Carcasses, Body Parts, and Tissue
- Infectious animal carcasses are placed in a red biohazard bag secured with a plastic tie and incinerated through the Animal Resources Center.
- All non-preserved carcasses should be stored in a freezer or cold storage area prior to disposal. Secure limbs and sharp protrusions so they do not puncture the bag.
Uncontaminated Laboratory Glassware and Broken Glass
Collect uncontaminated laboratory glassware and broken glass in designated rigid containers (separate from other waste) that will prevent cuts and punctures to personnel. Containers should be labeled “broken glass.” The rigid containers with broken glass (often a cardboard box) are taped shut and disposed of in the dumpster.
Chapter 15 - Transportation and Shipment of Biological Materials
The packaging and transportation/shipment of biological materials are subject to strict local, state, federal, and international regulations, which govern materials that are transported through the “public domain,” (i.e., roadways, airways, and sea lanes accessible to the public).
The intent of the packaging and transportation regulations is to prevent accidental exposure of personnel who may handle the material during its shipment. Therefore, certain general criteria apply to all possible transportation scenarios.
Transportation on Campus
- Staff transporting biological agents on campus must follow the Transporting Biological Agents Policy.
Packaging and Shipping Infectious Agents
The International Air Transport Association (IATA) and US Department of Transportation (DOT) regulate shipment of hazardous substances. The IATA regulations apply to all air transport, both domestic and international flights. Following IATA’s Dangerous Good Regulations (DGR), latest edition, ensures that a package will also meet US Department of Transportation requirements for ground transport.
All responsibilities for packaging and shipment of these agents have been assigned to the shipper. Only properly trained personnel may prepare infectious materials for transport. The following is only a summary of the requirements for packaging and shipping infectious agents and does not constitute proper training.
Definitions and Applicability
- Dangerous goods: any substance or material that is capable of posing an unreasonable risk to health, safety, and property when transported. Most infectious or biological materials are considered dangerous goods and therefore subject to shipping regulation.
- Infectious substances: substances which are known or reasonably expected to contain pathogens. Pathogens are defined as micro-organisms (including bacteria, viruses, rickettsia, parasites, fungi) and other agents, such as prions, which can cause disease in humans or animals.
For the purposes of shipping classification, infectious substances are broken into two categories:
Category A: an infectious substance which is transported in a form that, when exposure to it occurs, possesses the capability to cause permanent disability, life-threatening disease or fatal disease in otherwise healthy humans or animals.
Category B: an infectious substance that does not meet the criteria for inclusion in Category A.
Exempt Substances: the following substances are not required to follow the IATA or DOT regulations, but are still subject to MSU transport policies on campus:
- Materials that do not contain pathogens
- Materials that contain fully inactivated or neutralized pathogens
- Environmental samples that do not pose significant threat of infection (e.g., food, water, soil, or dust samples)
- Substances containing micro-organisms that are non-pathogenic to humans or animals
- Human or animal specimens in which there is minimal likelihood that pathogens are present
- Dried blood spots, fecal occult blood screening tests, blood or blood products intended for transfusion, and tissues or organs intended for transplantation.
The substance to be shipped must be classified as exempt from regulation, an exempt
patient specimen, Category A, or Category B infectious substance. Once classified,
proper shipping names and identification numbers can then be assigned to the material.
Exempt patient specimens do not require shipping names and identification numbers.
Category A and B materials are assigned the following names and numbers:
Category A: assign one of two identifiers:
- Infectious substance affecting humans: UN 2814
- Infectious substance affecting animals: UN 2900
Note: If a material infects both humans and animals, use the Infectious substance affecting human code, UN 2814.
Category B: Biological Substance, category B: UN 3373
All regulated infectious substances, including Category A, Category B, and exempt patient specimens, must be triple packaged:
- The innermost primary receptacle(s) is leak-proof.
- A leak-proof secondary receptacle with absorbent material placed between the primary and secondary receptacles to prevent the release of liquid during transport and to shield multiple primary receptacles from making contact.
- Rigid outer packaging
Shipments of Category A and Category B materials must be packaged according to IATA
Packing Instructions 602 and 650 respectively.
Shipments containing dry ice must follow IATA Packing Instruction 904.
Staff must refer to the current packing instructions to ensure the materials are packaged
correctly.
Those guidelines require the following:
- Shipments must be prepared in such a way that they arrive at their destination in good condition and present no hazard to persons or animals during shipment.
- Outer packaging must meet structural strength requirements and carry defined specification markings.
- Packages must have at least one surface with minimum dimensions of 100 mm x 100 mm.
- An itemized list of contents must be enclosed between the secondary container(s) and the outer packaging.
- All packages containing infectious substances must be marked durably and legibly on the outside of the package with the name and telephone number of a person responsible for the shipment.
- The shipper must make advance arrangements with the recipient and the operator to ensure the shipment can be transported and delivered without unnecessary delay.
- Substances shipped refrigerated or frozen must carry the refrigerant outside the secondary container or in the outer packaging or an overpack. If wet ice is used, the outside packaging or overpack must be leakproof. If dry ice is used, the packaging must permit the release of CO2 gas.
- Primary and secondary containers must retain their integrity across the full range of pressures and temperatures experienced under normal and loss-of-refrigerant conditions.
Labeling
Package labeling is in the form of standardized pictures that must be affixed to the outside. The color and design of each label is prescribed in the IATA regulations. All labels must be at least 50 mm on the smallest side. Refer to IATA Packing Instructions for specific requirements.
For the purposes of infectious substances, five different labels must be considered:
Category A:

Category B:

Dry Ice:
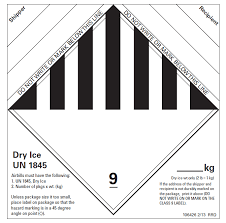

Cargo Aircraft Only: must be affixed if shipping volumes greater than 50 ml of a Category A substances
Orientation Arrows: if shipping liquids, two such labels must be affixed to the package, on opposing sides.

Markings
Markings are the words and numbers required to be on the outside of a package.
The following markings must be present on any package containing a Category A or Category B material:
- UN Number and Proper Shipping Name:
- UN 2814 Infectious substance affecting humans
- UN 2900 Infectious substance affecting animals
- Biological substance category B
- Contact Information requires the following:
- Name and telephone number of the responsible person
- 24-hour emergency telephone number in case of transportation emergency
- “To” and “from” information
If shipping a material under dry ice, the following additional marking is required:
- UN 1845 Dry Ice (the weight in kilograms of the dry ice present should also be noted)
- If shipping an exempt patient specimen, the only marking required is:
- Exempt Human Specimen or
- Exempt Animal Specimen
Training Requirements
Those involved in the packaging and shipping of infectious substances must undergo training every two years or when activities change. It is the department’s responsibility to ensure training is completed. The shipper is obligated to receive further qualification when shipping hazardous materials of a class or division where current training is insufficient.
Shipping Documents
Shipping papers describing the material in transit must accompany all shipments of dangerous goods. For ground transport, a Bill of Lading is required. For air transport, an Airway Bill takes the place of a Bill of Lading. However, air transport of a Category A material also requires that a Shipper’s Declaration of Dangerous Goods be filled out. The full and accurate completion of the Shipper’s Declaration is essential, as these are legal documents signed by the shipper, which creates a contract between the shipper and the carrier. The document must be accurate, legible, and neat and without any spelling errors.
- The declaration form must be completed in English.
- Three copies of the declaration must be completed. One copy will remain with the shipper. Two copies will be sent with the shipment. If the declaration is not a three-part NCR form, photocopies must be made.
Appendix A - Importation and Exportation of Etiologic Agents
Multidisciplinary and multi-institutional research is a common practice that involves collaboration among faculty from various institutions and countries. At times, it is necessary to share biological samples or materials with collaborators. Federal regulations strictly control the importation and exportation of etiologic agents. The following outlines two major requirements that must be followed.
All importation and exportation of etiologic agents must have a material transfer agreement (MTA) and be approved by the Biosafety Program.
CDC Etiologic Agent Import Permit Program
Etiologic agents are materials known or reasonably expected to contain a pathogen. This includes microorganisms and biological toxins that cause disease in humans and include bacteria, bacterial toxins, viruses, fungi, rickettsia, protozoans, and parasites. These disease-causing microorganisms may also be referred to as infectious agents. Arthropods and other organisms that transmit pathogens to animals (including humans) are called vectors.
Etiologic agents, vectors, and materials containing etiologic agents are recognized as hazardous materials. Materials containing etiologic agents are regularly transported from one location to another by common land and air carriers. Materials containing etiologic agents must be appropriately packaged to prevent breakage or leakage to avoid exposing the package contents to package handlers, transporters, and the public. Materials containing etiologic agents must be packaged, labeled, and transported in accordance with all applicable regulations. Material containing etiologic agents being imported into the United States must be accompanied by a U.S. Public Health Service importation permit.
Importation Permits
Importation permits are issued only to the importer, who must be located in the United States. The importation permit, with the proper packaging and labeling, will expedite clearance of the package of infectious materials through the U.S. Public Health Service Division of Quarantine and release by U.S. Customs.
The importer is legally responsible for ensuring that the foreign personnel package, label, and ship the infectious materials according to federal and international regulations. Shipping labels with the universal biohazard symbol, the importer’s address, the permit number, and the expiration date are also issued to the importer with the permit. The importer must send the labels and one or more copies of the permit to the shipper. The permit and labels inform the U.S. Customs Service and U.S. Division of Quarantine personnel of the package contents.
Utilize the CDC IPP website for permitting questions and forms.
Federal Regulation
The importation of etiologic agents is governed by the following federal regulation: USPHS 42 CFR - Part 71 Foreign Quarantine. Part 71.54 Etiologic agents, hosts, and vectors.
A person may not import into the United States any infectious biological agent, infectious substance, or vector unless:
(1) It is accompanied by a permit issued by the Centers for Disease Control and Prevention (CDC). The possession of a permit issued by the CDC does not satisfy permitting requirements placed on materials by the U.S. Department of Agriculture that may pose hazards to agriculture or agricultural production in addition to hazards to human health.
(2) The importer is in compliance with all of the permit requirements and conditions that are outlined in the permit issued by the CDC.
(3) The importer has implemented biosafety measures commensurate with the hazard posed by the infectious biological agent, infectious substance, and/or vector to be imported, and the level of risk given its intended use.
(4) The importer takes measures to help ensure that the shipper complies with all applicable legal requirements concerning the packaging, labeling, and shipment of infectious substances.
Permitted materials may not be distributed or transported to other users unless accompanied by a separate permit.
Items Requiring Permits
To confirm if a permit is required, utilize the CDC’s IPP e-Tool.
Etiologic agents
An import permit is needed for any infectious agent known or suspected to cause disease in humans.
Biological materials
Unsterilized specimens of human and animal tissues (such as blood, body discharges, fluids, excretions, or similar material) containing an infectious or etiologic agent require a permit in order to be imported.
Hosts and vectors
-
Animals: any animal known or suspected of being infected with an organism capable of causing disease in humans may require a permit issued by CDC. Importation of live turtles of less than 4 inches in shell length and live nonhuman primates is regulated by the CDC’s Division of Global Migration and Quarantine (http://www.cdc.gov/ncidod/dq/).
-
Bats: all live bats require an import permit from the CDC and the U.S. Department of Interior, Fish and Wildlife Services. The application for a CDC import permit for live exotic bats is at CDC Importation of Animals website (http://www.cdc.gov/ncidod/dq/).
-
Arthropods: any living insect or other arthropod that is known or suspected of containing an etiologic agent (human pathogen) requires a CDC import permit.
-
Snails: snail species capable of transmitting a human pathogen require a CDC permit.
Packaging Requirements
Infectious materials imported into this country must be packaged to withstand breakage and leakage of contents and be labeled, as specified in the following federal regulations:
- USPHS 42 CFR Part 72 - Interstate Shipment of Etiologic Agents
- DOT 49 CFR PART 173 - Transportation of Etiologic Agents
- For international shipments, the International Air Transport Association’s (IATA) Dangerous Goods Regulations should be consulted.
Other Permits
-
USDA and APHIS permits are required for infectious agents of livestock and biological materials containing animal material. Tissue culture materials and suspensions of cell culture-grown viruses or other etiologic agents containing growth stimulants of bovine or other livestock origins are controlled by the USDA because of the potential risk of introduction of exotic animal diseases into the United States. For more information, contact USDA/APHIS at their website, http://www.aphis.usda.gov/animal_health/.
Principal Investigators must submit USDA/APHIS permit applications via the Biosafety Website.
- U.S. Fish and Wildlife Service permits are required for certain live animals, including bats. For more information, call (800) 344-WILD or visit their website, http://www.fws.gov/.
- Individuals wishing to import select agents and toxins must be registered with the CDC’s Select Agent Program in accordance with 42 CFR Part 73 (Possession, Use, and Transfer of Select Agents and Toxins; Interim Final Rule) for the select agent(s) and toxin(s) listed on the import permit application. Also, in accordance with 42 CFR Part 73.16(a), an APHIS/CDC Form 2 must be completed and submitted to the CDC Select Agent Program and granted approval prior to the shipment of the select agents or toxins under the import permit. Additional information can be found at http://www.cdc.gov/od/sap.
A permit issued under this part is not required for an item if:
(1) It is a biological agent listed in 42 CFR Part 73 as a select agent and its importation has been authorized in accordance with 42 CFR 73.16 or 9 CFR 121.16.
(2) With the exception of bat or nonhuman primate specimens, it is a diagnostic specimen not known by the importer to contain, or suspected by the importer of containing, an infectious biological agent and is accompanied by an importer certification statement confirming that the material is not known to contain or suspected of containing an infectious biological agent, or has been rendered noninfectious.
(3) With the exception of live bats or bat or nonhuman primate products, it is an animal or animal product being imported for educational, exhibition, or scientific purposes and is accompanied by documentation confirming that the animal or animal product is not known to contain (or suspected of containing) an infectious biological agent or has been rendered noninfectious.
(4) It consists only of nucleic acids that cannot produce infectious forms of any infectious biological agent and the specimen is accompanied by an importer certification statement confirming that the material is not known to contain or suspected of containing an infectious biological agent.
(5) It is a product that is cleared, approved, licensed, or otherwise authorized under any of the following laws:
(i) The Federal Food, Drug, and Cosmetic Act (21 U.S.C. 301 et seq.), or
(ii) Section 351 of the Public Health Service Act pertaining to biological products (42 U.S.C. 262), or
(iii) The Virus-Serum-Toxin Act (21 U.S.C. 151-159).
(6) It is an animal or animal product listed in 42 CFR Part 71 and its importation has been authorized in accordance with 42 CFR 71.52, 71.53, or 71.56.
Exportation of Infectious Materials
The export of a wide variety of etiologic agents of human, plant, and animal diseases may require a license from the Department of Commerce. To determine if a license is necessary, visit http://www.bis.doc.gov/Licensing/.
The MSU Export Control Program will assist you in determining whether an export license is required for your shipment. If an export license is required, then you should anticipate and plan for a non-trivial delay while your license application is prepared by MSU, and then reviewed by the Department of Commerce.
Export control regulations are rather complex and may have multi-agency jurisdictions that must approve activities. The Department of Commerce regulations state that:
“Activities subject to the Export Administration Regulations (EAR) may also be controlled under export-related programs administered by other agencies. Items and activities subject to the EAR are not necessarily exempted from the control programs of other agencies.”
Please contact both the Export Control and the Biosafety Program as soon as possible if you intend to export any biological materials. Together, these MSU resources can help assist you in navigating the required processes and ensuring both you and MSU remain legally compliant with applicable federal law.
Appendix B - Laboratory Ventilation and Containment for Biosafety
Categories of Laboratory-ventilated containment equipment.
Chemical Fume Hoods (CFH), Laminar Flow Clean Benches (LFCB), and Biological Safety Cabinets (BSC)
|
|
Personnel
|
Product
|
Environmental
|
|---|---|---|---|
|
Chemical Fume Hoods
|
|
|
|
|
Laminar Flow Clean Benches
|
|
|
|
|
Class I Biological Safety Cabinets
|
|
|
|
|
Class II Biological Safety Cabinet
|
|
|
|
Laboratory Chemical (“Fume”) Hoods
Traditional laboratory chemical (or fume) hoods are designed to capture, contain and exhaust chemical fumes or vapors and pull them away from the worker. Although the inward flow of air protects the user, chemical hoods do not protect the product or the environment (there is no HEPA filter on the exhaust).
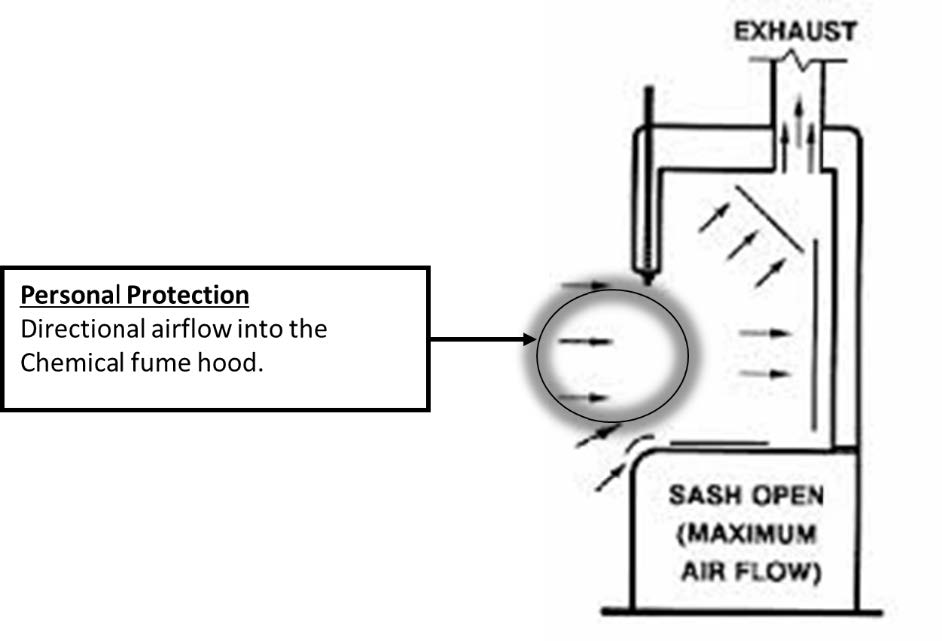
Laminar Flow Hood/Clean Bench
With horizontal laminar flow hoods (LFH), HEPA-filtered air flows horizontally across the workspace directly toward the user.
LFHs provide product protection against microbial contamination, but they do not provide personal or environmental protection. The horizontal flow of air will blow biological agents directly toward the user and into the laboratory. LFHs are not a biological safety cabinet, and they must not be used with any materials (biological, chemical, or radiological) requiring containment for protection of personnel or the environment.
LFHs are acceptable for tissue culture work only with cell lines considered to represent low risk (BSL-1 agents) to laboratory workers (including immunocompromised individuals who may frequent the lab). Human cell lines and nonhuman primate cell lines are considered to be BSL-2 agents and cannot be used in a LFH.
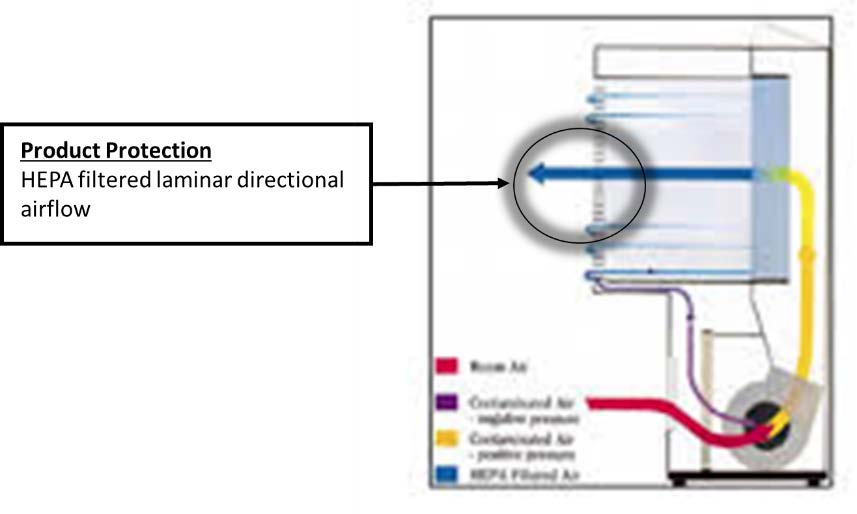
Biological Safety Cabinets
A Biological safety cabinet (BSC) is a necessary containment device when working with potentially infectious materials. BSCs are divided into Class I, II, and III (see schematic below). Class II BSCs are subdivided into type A and type B. All BSCs provide personnel and environmental protection, with Class II BSCs also providing product protection.
- Personnel protection is achieved by inward airflow through the front of the
- Product protection is achieved by downward HEPA-filtered airflow from the top of the
- Environmental protection is achieved by HEPA filtration of exhaust
Class I BSC
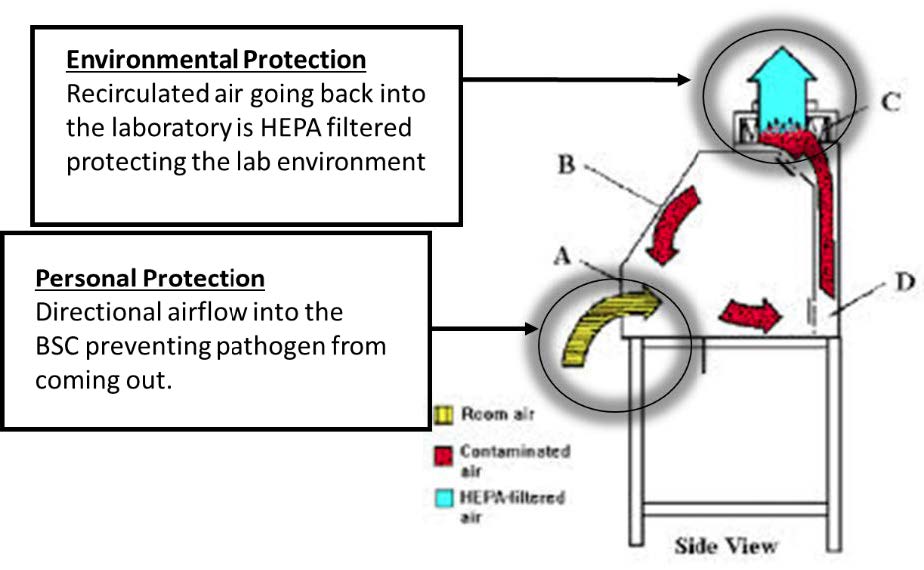
Class II BSC
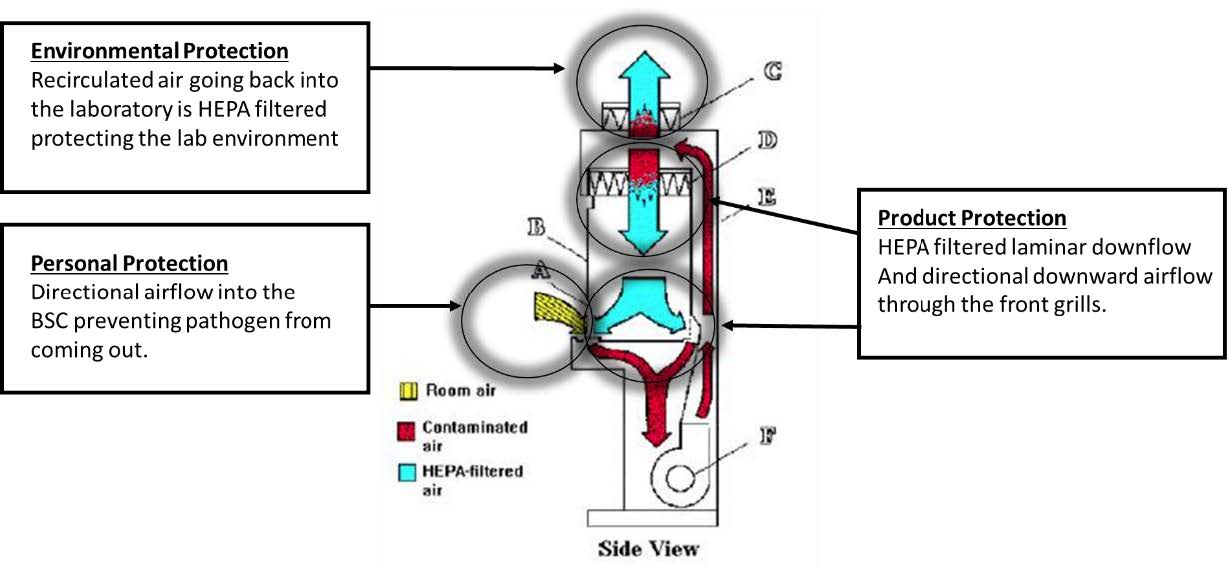
Certification of BSCs
Field certification of BSCs is required to ensure that the cabinet still performs as it did when it obtained NSF certification at the factory. NIH requires field certification under the following circumstances: (1) upon installation of a new BSC; (2) annually thereafter; (3) after repair or maintenance is performed; and (4) after the BSC is relocated.
CDC recommends that BSCs be recertified annually to ensure for proper function. They will also be recertified after being moved to ensure that they have not been damaged. Laboratories are responsible for ensuring that the BSCs are recertified in a timely manner. The contact information to reach the contractor is indicated on the certification sticker affixed on the front of the BSC.
NSF/ANSI 49 provides criteria for construction of BSCs, testing by manufacturers (including biological containment testing), and field certification. NSF has also established a certification program for field certifiers to ensure a minimum level of competency and professionalism. It is recommended that NSF field certifiers be used for field certification of BSCs.
|
Biosafety Cabinet
|
Chemical Fume Hood
|
Laminar Flood Hood
|
|---|---|---|
|
Primarily designed to protect against exposure to particulates and aerosols from biological agents. Provides product, personnel and environmental protection. |
Ventilated, enclosed work space intended to capture, contain and exhaust harmful or dangerous chemical fumes, vapors and particulate matter outside the laboratory. |
Primarily designed to provide a sterile work environment. Does not provide any protection to personnel or environment |
|
Should only be used for work with infectious agents or for the capture of nuisance dust and allergens from bulk operations such as animal cage changing or dumping |
The most suitable choice for chemical use including preparation of hazardous drugs and volatile anesthetic gases used for animal anesthesia and/or euthanasia |
Should only be used for work with non-infectious materials, such as media preparation. |
|
Never use with:
|
Never use with:
|
Never use with:
|
Appendix C - Biosafety Level 2 (BSL-2) Requirements
Biosafety Level 2 (BSL-2) is suitable for work with agents associated with human disease and pose moderate hazards to personnel and the environment.
For example:
- Microorganisms of low biohazard potential, such as those in Risk Group 2 or BSL-2.
- Recombinant DNA activity requiring BSL-2 physical containment including animal studies that involve the construction of transgenic animals.
- Non-recombinant cell and/or tissue culture systems that require this level of containment.
• Oncogenic viral systems classified as low risk. - Production activities with Risk Group 1 organisms.
The following are procedures are used for BSL-2 containment. They are based on the recommendation of the Biosafety in Microbiological and Biomedical Laboratories (BMBL) 6th Edition.
Standard Microbiological Practices
- The laboratory supervisor enforces the institutional policies that control safety in and access to the laboratory.
- The laboratory supervisor ensures that laboratory personnel receive appropriate training regarding their duties, potential hazards, manipulations of infectious agents, necessary precautions to minimize exposures, and hazard/exposure evaluation procedures (e.g., physical hazards, splashes, aerosolization) and that appropriate records are maintained. Personnel receive annual updates and additional training when equipment, procedures, or policies change. All persons entering the facility are advised of the potential hazards, are instructed on the appropriate safeguards, and read and follow instructions on practices and procedures. An institutional policy regarding visitor training, occupational health requirements, and safety communication is considered.
- Personal health status may affect an individual’s susceptibility to infection and ability to receive available immunizations or prophylactic interventions. Therefore, all personnel, and particularly those of reproductive age and/or those having conditions that may predispose them to increased risk for infection (e.g., organ transplant, medical immunosuppressive agents), are provided information regarding immune competence and susceptibility to infectious agents. Individuals having such conditions are encouraged to self-identify to the institution’s healthcare provider for appropriate counseling and guidance.
- A safety manual specific to the facility is prepared or adopted in consultation with
the facility director and appropriate safety professionals. The safety manual is available,
accessible, and periodically reviewed and updated as necessary.
- The safety manual contains sufficient information to describe the biosafety and containment procedures for the organisms and biological materials in use,appropriate agent-specific decontamination methods, and the work performed.
- The safety manual contains or references protocols for emergency situations, including exposures, medical emergencies, facility malfunctions, and other potential emergencies. Training in emergency response procedures is provided to emergency response personnel and other responsible staff according to institutional policies.
- A sign incorporating the universal biohazard symbol is posted at the entrance to the laboratory when infectious materials are present. Posted information includes: the laboratory’s Biosafety Level, the supervisor’s or other responsible personnel’s name and telephone number, PPE requirements, general occupational health requirements (e.g., immunizations, respiratory protection), and required procedures for entering and exiting the laboratory. Agent information is posted in accordance with the institutional policy
- Long hair is restrained so that it cannot contact hands, specimens, containers, or equipment.
- Gloves are worn to protect hands from exposure to hazardous materials.
- Glove selection is based on an appropriate risk assessment.
- Gloves are not worn outside the laboratory.
- Change gloves when contaminated, glove integrity is compromised, or when otherwise necessary.
- Do not wash or reuse disposable gloves, and dispose of used gloves with other contaminated laboratory waste.
- Gloves and other PPE are removed in a manner that minimizes personal contamination and transfer of infectiousinfectious materials and/or animals are housed or manipulated.
- Persons wash their hands after working with potentially hazardous materials and before leaving the laboratory.
- Eating, drinking, smoking, handling contact lenses, applying cosmetics, and storing food for human consumption are not permitted in laboratory areas. Food is stored outside the laboratory area.
- Mouth pipetting is prohibited. Mechanical pipetting devices are used.
- Policies for the safe handling of sharps, such as needles, scalpels, pipettes, and
broken glassware are developed, implemented, and followed; policies are consistent
with applicable state, federal, and local requirements. Whenever practical, laboratory
supervisors adopt improved engineering and work practice controls that reduce risk
of sharps injuries. Precautions are always taken with sharp items. These include:
- Plasticware is substituted for glassware whenever possible.
- Use of needles and syringes or other sharp instruments materials outside of the areas
where is limited in the laboratory and is restricted to situations where there is
no alternative (e.g., parenteral injection, blood collection, or aspiration of fluids
from laboratory animals or diaphragm bottles). Active or passive needle-based safety
devices are to be used whenever possible.
- Uncapping of needles is performed in such a manner to reduce the potential for recoil causing an accidental needlestick.
- Needles are not bent, sheared, broken, recapped, removed from disposable syringes, or otherwise manipulated by hand before disposal.
- If absolutely necessary to remove a needle from a syringe (e.g., to prevent lysing blood cells) or recap a needle (e.g., loading syringes in one room and injecting animals in another), a hands-free device or comparable safety procedure must be used (e.g., a needle remover on a sharps container, the use of forceps to hold the cap when recapping a needle).
- Used, disposable needles and syringes are carefully placed in puncture-resistant containers used for sharps disposal immediately after use. The sharps disposal container is located as close to the point of use as possible.
- Non-disposable sharps are placed in a hard-walled container for transport to a processing area for decontamination, preferably by autoclaving.
- Broken glassware is not handled directly. Instead, it is removed using a brush and dustpan, tongs, or forceps.
- Perform all procedures to minimize the creation of splashes and/or aerosols.
- Decontaminate work surfaces after completion of work and after any spill or splash of potentially infectious material with appropriate disinfectant. Spills involving infectious materials are contained, decontaminated, and cleaned up by staff who are properly trained and equipped to work with infectious material. A spill procedure is developed and posted within the laboratory.
- Decontaminate all cultures, stocks, and other potentially infectious materials before
disposal using an effective method, consistent with applicable institutional, local,
and state requirements. Depending on where the decontamination will be performed,
the following methods are used prior to transport:
- Materials to be decontaminated outside of the immediate laboratory are placed in a durable, leak-proof container and secured for transport. For infectious materials, the outer surface of the container is disinfected prior to moving materials and the transport container has a universal biohazard label.
- Materials to be removed from the facility for decontamination are packed in accordance with applicable local, state, and federal regulations.
- An effective integrated pest management program is implemented.
- Animals and plants not associated with the work being performed are not permitted in the laboratory
Special Practices
- Access to the laboratory is controlled when work is being conducted.
- The laboratory supervisor is responsible for ensuring that laboratory personnel demonstrate proficiency in standard microbiological practices and techniques for working with agents requiring BSL-2 containment.
- Laboratory personnel are provided medical surveillance, as appropriate, and offered available immunizations for agents handled or potentially present in the laboratory.
- Properly maintained BSCs or other physical containment devices are used, when possible,
whenever:
- Procedures with a potential for creating infectious aerosols or splashes are conducted. These include pipetting, centrifuging, grinding, blending, shaking, mixing, sonicating, opening containers of infectious materials, inoculating animals intranasally, and harvesting infected tissues from animals or eggs.
- High concentrations or large volumes of infectious agents are used. Such materials may be centrifuged in the open laboratory using sealed rotors or centrifuge safety cups with loading and unloading of the rotors and centrifuge safety cups in the BSC or another containment device.
- If it is not possible to perform a procedure within a BSC or other physical containment device, a combination of appropriate personal protective equipment and administrative controls are used, based on a risk assessment.
- Laboratory equipment is decontaminated routinely; after spills, splashes, or other potential contamination; and before repair, maintenance, or removal from the laboratory.
- A method for decontaminating all laboratory waste is available (e.g., autoclave, chemical disinfection, incineration, or other validated decontamination method).
- Incidents that may result in exposure to infectious materials are immediately evaluated per institutional policies. All such incidents are reported to the laboratory supervisor and any other personnel designated by the institution. Appropriate records are maintained.
Safety Equipment (Primary Barriers)
- Protective laboratory coats, gowns, or uniforms designated for laboratory use are worn while working with hazardous materials and removed before leaving for non-laboratory areas (e.g., cafeteria, library, and administrative offices). Protective clothing is disposed of appropriately or deposited for laundering by the institution. Laboratory clothing is not taken home.
- Eye protection and face protection (e.g., safety glasses, goggles, mask, face shield or other splatter guard) are used for manipulations or activities that may result in splashes or sprays of infectious or other hazardous materials. Eye protection and face protection are disposed of with other contaminated laboratory waste or decontaminated after use.
- The risk assessment considers whether respiratory protection is needed for the work with hazardous materials. If needed, relevant staff are enrolled in a properly constituted respiratory protection program.
- In circumstances where research animals are present in the laboratory, the risk assessment considers appropriate eye, face, and respiratory protection, as well as potential animal allergens.
Laboratory Facilities (Secondary Barriers)
- Laboratory doors are self-closing and have locks in accordance with the institutional policies.
- Laboratories have a sink for handwashing. It should be located near the exit door.
- An eyewash station is readily available in the laboratory.
- The laboratory is designed so that it can be easily cleaned.
- Carpets and rugs in laboratories are not appropriate.
- Spaces between benches, cabinets, and equipment are accessible for cleaning.
- Laboratory furniture can support anticipated loads and uses.
- Benchtops are impervious to water and resistant to heat, organic solvents, acids, alkalis, and other chemicals.
- Chairs used in laboratory work are covered with a non-porous material that can be easily cleaned and decontaminated with appropriate disinfectant.
- Laboratory windows that open to the exterior are not recommended. However, if a laboratory does have windows that open to the exterior, they are fitted with screens.
- Illumination is adequate for all activities and avoids reflections and glarethat could impede vision.
- Vacuum lines in use are protected with liquid disinfectant traps and in-line HEPA filters or their equivalent.
- There are no specific requirements for ventilation systems. However, the planning of new facilities considers mechanical ventilation systems that provide an inward flow of air without recirculation to spaces outside of the laboratory.
- BSCs and other primary containment barrier systems are installed and operated in a
manner to ensure their effectiveness.
- BSCs are installed so that fluctuations of the room air supply and exhaust do not interfere with proper operations. BSCs are located away from doors, windows that can be opened, heavily traveled laboratory areas, and other possible airflow disruptions.
- BSCs can be connected to the laboratory exhaust system by either a canopy connection (Class IIA only) or directly exhausted to the outside through a hard connection (Class IIB, IIC, or III). Class IIA or IIC BSC exhaust can be safely recirculated back into the laboratory environment if no volatile toxic chemicals are used in the cabinet.
- BSCs are certified at least annually to ensure correct performance.
Procedures for Receiving and Inspecting Samples
- The PI will designate a responsible person for the purchase of all infectious materials to be used in the BSL-2 lab.
- Infectious materials will be shipped to the laboratory in accordance with the appropriate Department of Transportation (DOT) and the International Air Transportation Association (IATA) standards for shipping of infectious biological materials.
- Upon receipt of the package, it will be placed on a tray covered with absorbent material and opened in the Biological Safety Cabinet to prevent any potential exposure to personnel in case the container leaked during transport.
- Personnel assigned to open packages will wear lab coat, gloves, and eye protection.
- If any containers are found to be damaged, leaking or otherwise contaminated, they will be immediately isolated into a plastic bag along with all packaging materials. The spill will be disinfected and cleaned up. The Principal Investigator, lab director or designee will be notified immediately. The incident will be reported to ORC/SRM and as necessary, to appropriate agencies.
- If, after inspection, the samples are intact, they can be placed into labeled secondary containers (unbreakable plastic containers or metal tubes) and then transferred to a storage area.
- Only staff who are authorized to do so can remove samples from storage. Removal and use of all such materials must be entered into the inventor
