Montana IPM Bulletin, Spring 2016

The Montana IPM Bulletin presents critical pest management and pesticide education articles for Montana homeowners, pesticide applicators, farmers and ranchers. These articles are designed to deliver timely updates from an unbiased perspective that are specific to Montana. This is a cooperative effort between Montana State University pesticide education and integrated pest management programs.
In this Issue
- Pestweb: The Montana Orange Wheat Blossom Midge Monitoring Project
- EPA Proposing Strong Steps to Prevent Poisoning from Paraquat
- All Thistles Are Not Created Equal
- Pest Management Tool Kit
- Ask the Expert
- Glyphosate-Resistant Conyza canadensis (marestail, horseweed) Confirmed in NE Montana
- Meet Your Specialist
- Acknowledgments
Pestweb: The Montana Orange Wheat Blossom Midge Monitoring Project
by Cecil Tharp, Montana State University Pesticide Education Specialist

Figure 1. Orange wheat blossom midge larvae feeding on a developing wheat kernel. Photo credit NW Agricultural Research Center.
The Orange Wheat Blossom Midge (Figure 1), hereafter referred to as wheat midge, first caused economic damage during 2006 in Flathead County, with spring wheat yield losses estimated at 1.5 million dollars (Figure 2). Since the initial outbreak, the wheat midge was also found in Lake County, but its exact distribution in the rest of the state was largely unknown. As such, a pilot project was initiated to monitor for the presence of this pest in the Golden Triangle area.
Pondera County first reported low wheat midge numbers in 2008, but it wasn’t until 2013 that economic levels of this pest were found, and over 12,000 acres had to be treated. The season of 2013 also found low wheat midge populations in Chouteau, Glacier, Liberty and Toole Counties, while wheat midge damage was suspect in Daniels County. This dramatic increase in the distribution of wheat midge served as the initiative behind the development of the MSU Pestweb website.
The MSU Pestweb website is partially funded by a grant from the Montana Wheat and Barley Committee and serves as an early warning system to help producers track wheat midge adult emergence and distribution throughout the state of Montana on a field scale basis.

Figure 2. Damage to wheat kernels by orange wheat blossom midge larvae. Photo credit NW Agricultural Research Center.
2015 Activities
Monitoring for the wheat midge was accomplished using pheromone traps. These traps attract wheat midges and contain them so researchers can estimate their population sizes at sites across Montana. Faculty at six Research Centers participated in monitoring fields. The Research Centers also served as the regional hubs for distributing over 100 pheromone traps to 31 MSU Extension agents. In turn, some Extension agents served as the distribution point for an additional 200 pheromone traps for use by area farmers and crop consultants, while some individuals purchased traps directly from suppliers.
In all, 44 volunteers in 31 counties aided in monitoring for the presence of the wheat midge in a total of 275 fields during 2015. The information the volunteers obtained was posted on the MSU Pestweb website (pestweb.montana.edu). This information was made available to the general public so that small grain growers throughout the state were able to see if wheat midge populations were present in their immediate area and to determine if the numbers warranted scouting their fields.
Results
A total of 17 counties reported presence of the wheat midge in 2015 (Table 1). Thus far, wheat midge infestations appear to be concentrated in the northwestern, northcentral and northeastern tier of counties (Figure 3). Wheat midge had previously been reported in Blaine, Chouteau, Daniels, Flathead, Glacier, Hill, Lake, Liberty, Phillips, Pondera, Prairie, Richland, Roosevelt, Sheridan, Teton, Toole, Valley and Wibaux Counties, and the 2015 monitoring project verified this fact. However, three additional counties have now documented presence of the wheat midge as a result of the 2015 efforts. The new counties include, Garfield, Judith Basin and McCone. These results indicate wheat midge populations are spreading throughout Montana, and that continued vigilance is warranted.
Use the button below to show or hide the Orange Wheat Blossom Midge monitoring table (Table 1).
Below is a quick-reference data table for the testing detailed in this article. The table consists of 4 columns, the first contains the participating county; the proceeding columns represent the number of wheat midge traps that were set, followed by the total count of trapped midges, and then the average midge count per trap.
|
Participating County
|
Number of Traps
|
Total Trap Count
|
Average Count per Trap
|
|---|---|---|---|
|
Flathead
|
55
|
34403
|
626
|
|
Pondera
|
44
|
14373
|
327
|
|
Sheridan
|
4
|
5541
|
1385
|
|
Valley
|
13
|
4605
|
354
|
|
Richland
|
6
|
1843
|
307
|
|
Liberty
|
25
|
892
|
36
|
|
Roosevelt
|
4
|
799
|
200
|
|
McCone
|
3
|
706
|
235
|
|
Daniels
|
5
|
700
|
140
|
|
Phillips
|
3
|
471
|
157
|
|
Glacier
|
15
|
351
|
23
|
|
Lake
|
14
|
299
|
21
|
|
Prairie
|
4
|
208
|
52
|
|
Teton
|
7
|
41
|
6
|
|
Garfield
|
4
|
29
|
7
|
|
Toole
|
3
|
5
|
2
|
|
Judith Basin
|
5
|
1
|
0
|
|
Bighorn
|
1
|
0
|
0
|
|
Blaine
|
6
|
0
|
0
|
|
Broadwater
|
2
|
0
|
0
|
|
Carter
|
5
|
0
|
0
|
|
Cascade
|
2
|
0
|
0
|
|
Chouteau
|
14
|
0
|
0
|
|
Custer
|
2
|
0
|
0
|
|
Fergus
|
5
|
0
|
0
|
|
Gallatin
|
4
|
0
|
0
|
|
Hill
|
10
|
0
|
0
|
|
Ravalli
|
4
|
0
|
0
|
|
Treasure
|
1
|
0
|
0
|
|
Yellowstone
|
3
|
0
|
0
|
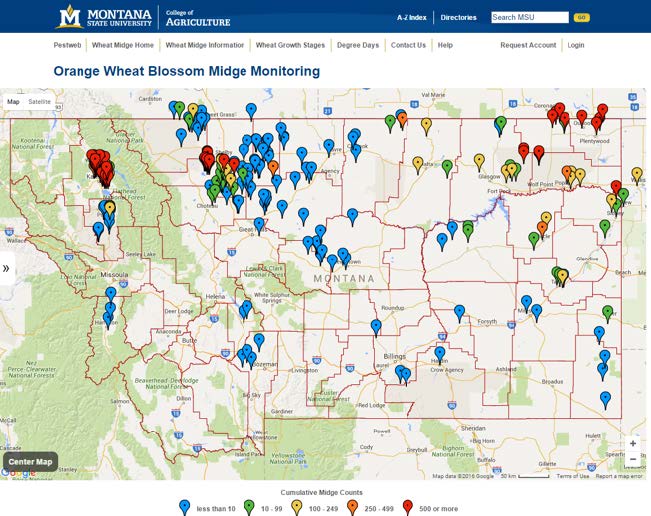
Figure 3. Map showing monitoring traps and intensity of infestations
For more information about the Orange Wheat Blossom Midge Monitoring Project, the new wheat midge MontGuide, or Pestweb, contact your local MSU Extension agent, your local MSU Agricultural Research Center, or the following individuals: Brooke Bohannon, Dan Picard, Gadi Reddy, Bob Stougaard, or Kevin Wanner.
EPA Proposing Strong Steps to Prevent Poisoning from Paraquat
by Cecil Tharp, Montana State University Pesticide Education Specialist
Paraquat is one of the most widely used herbicides in the United States, commonly sold in such formulations as Devour, Firestorm, Helmquat 3SL, Gramoxone SL, Cyclone SL 2.0, Bonedry, Willowood Paraquat 3SL, Paraquat Concentrate and Parazone 3SL Herbicide. Paraquat is a photosynthesis inhibitor and acts as a non-selective contact herbicide.
Use of paraquat in Montana has increased in recent years due to glyphosate resistance in kochia. The pesticide active ingredient, paraquat, enables applicators to mix their modes of action in an effort to reduce glyphosate resistance while managing a wide array of weeds. Paraquat also is useful as a desiccation aid in various crops.
Since paraquat is a highly toxic active ingredient that can be fatal at extremely low doses, the Environmental Protection Agency (EPA) is reviewing the implications of widespread use of this product. According to EPA, 17 deaths have been reported since 2000. This number includes three children that accidentally ingested paraquat as a result of the pesticide being transferred to beverage containers. Even a sip is fatal to a child, and there is no antidote. The EPA reports three additional pesticide worker deaths and many severe injuries caused by paraquat absorption through the skin or eyes.
-
New closed-system packaging designed to allow removal of pesticide only into proper application equipment
-
Special training for certified applicators using paraquat
-
Changes to the pesticide label to highlight toxicity and risks
-
Prohibiting application from hand-held and backpack equipment
-
Restricting use to certified pesticide applicators only (individuals working under the supervision of a certified applicator would be prohibited from using paraquat)
Submitting Comments on this Proposal
EPA is asking for public comment over a 60-day period. Comments must be received on or before May 9, 2016. Submit your comments, identified by docket identification (ID) number EPA-HQ- OPP-2011-0855, by one of the following methods:
-
Federal eRulemaking Portal: Go to docket EPA-HQ-OPP-2011-0855 on regulations.gov. Do not submit electronically any information you consider to be confidential business information or other information whose disclosure is restricted by statute.
- Mail: OPP Docket, Environmental Protection Agency Docket Center (EPA/ DC), (28221T), 1200 Pennsylvania Ave. N.W., Washington, DC 20460-0001.
- Hand Delivery: To make special arrangements for hand delivery or delivery of boxed information, please follow EPA instructions.
Precautions when Using Paraquat Products
Table 2. Personal Protective Equipment Requirements for Paraquat Concentrate
Applicators and other handlers (other than mixers and loaders) must wear:
- Long-sleeved shirt and long pants
- Chemical resistant gloves - Category A (e.g. barrier laminate, butyl rubber, nitrile rubber, neoprene rubber, natural rubber, polyethylene, polyvinyl chloride (PVC) or viton)
- Shoes plus socks
- Protective eyewear
- NIOSH-approved particulate filtering respirator equipped with N, R or P class filter media. The respirator should have a NIOSH approval number prefix TC-84A. It is recommended that you require that respirator wearer to be fit tested, and trained in the use, maintenance and limitations of the respirator.
Mixers and Loaders Must Wear:
- Long-sleeved shirt and long pants
- Chemical resistant gloves - Category A (e.g., barrier laminate, butyl rubber, nitrile
rubber, neoprene rubber,
natural rubber, polyethylene, polyvinyl chloride (PVC) or viton ) - Shoes plus socks
- Chemical resistant apron
- Face shield
- NIOSH-approved particulate filtering respirator equipped with N, R or P class filter
media. The respirator
should have a NIOSH approval number prefix TC-84A. It is recommended that you require that respirator
wearer to be fit tested, and trained in the use, maintenance and limitations of the respirator.
Applicators should use paraquat products with care as it is classified as a category 1 substance with the signal word ‘Danger – Poison.’ This signal word implies that this pesticide product is highly toxic through multiple routes of entry. When using paraquat products applicators should remember to:
- Have buffers between the pesticide application and sensitive areas (livestock, people, and pets).
- Follow all re-entry and pre-harvest requirements on the pesticide product label.
- Wear proper personal protective equipment.

Adjusting a regulator while wearing PPE. Photo by Cecil Tharp, MSU, 2004
Personal protective equipment (PPE) requirements are usually on the first or second page of the product label under Precautionary Statements. Table 2 represents the PPE requirements for applicators while using the paraquat formulation known as Paraquat Concentrate. This includes protective eyewear, long sleeve shirt and pants, protective eyewear, chemically resistant gloves and the use of a NIOSH approved particulate filtering respirator with any N, R or P filter with an approval prefix of TC-84A. Mixers and loaders must also wear a chemical resistant apron and face shield (Table 2).
Further Information
For questions regarding implications in Montana, contact Cecil Tharp, Pesticide Education Specialist (406-994-5067). For technical questions or further details regarding commenting, contact Michelle Arling.
All Thistles Are Not Created Equal
by Noelle Orloff, Plant Identification Diagnostician, and Jane Mangold, Rangeland Weed Specialist

Figure 4. Meadow or elk thistle (Cirsium scariosum). Photo by Matt Lavin, Montana State University
The Montana countryside is turning green and soon the hillsides will be dotted with colorful wildflowers, including thistles. In Montana, we have both exotic weedy thistles and beneficial thistles native to our state. The most visible and abundant thistle species are often exotic thistles like Canada thistle (Cirsium arvense) and bull thistle (Cirsium vulgare). These exotic thistles are often invasive and included on state and county noxious weed lists (Table 3). While you may think, “the only good thistle is a dead thistle,” thistles native to Montana such as elk thistle (Cirsium scariosum) (Figure 4) are better of alive than dead due to their benefits including beautiful flowers and foliage and resources they provide for wildlife, especially pollinators. From an economics perspective, it makes sense to focus limited resources on controlling only those thistles that may pose a true threat, namely exotic, invasive thistles.
Fifteen thistle species grow in Montana. Ten of these species are native while five are exotic and considered weedy or invasive (Table 3). Identifying exotic thistles and differentiating them from natives before attempting to control them with herbicides or by other means is critical. Invasive exotic thistles can spread quickly and form dense stands with disturbance (Figure 5), they have poor forage value, and their sharp spines can limit recreational activities and injure livestock. Compared to exotic thistles, native thistles do not spread quickly with disturbance, are rarely or never reported as invasive, and are important for wildlife. For example, birds eat thistle seed, and some may time their nesting around thistle flowering, using plumes on seeds to line their nests. Bees, wasps, flies and beetles feed on thistle pollen and become food sources for other wildlife. For some large ungulates like elk, native thistles are a source of forage.
Use the button below to show or hide the thistle species table (Table 3).
Table 3. Fifteen thistle species in Montana. The table consists of 2 columns, the first contains the thistle species, and the second indicates whether it is native or invasive in Montana.
|
Species
|
Native or Exotic
|
|---|---|
|
Bull thistle |
Exotic
|
|
Canada thistle* |
Exotic
|
|
Clustered thistle |
Native
|
|
Eaton’s thistle |
Native
|
|
Flodman’s thistle |
Native
|
|
Graygreen thistle
(Cirsium canovirens) |
Native
|
|
Longstyle thistle |
Native
|
|
Meadow or Elk thistle |
Native
|
|
Musk thistle** |
Exotic
|
|
Plumeless thistle** |
Exotic
|
|
Prairie thistle |
Native
|
|
Scotch thistle** |
Exotic
|
|
Wavyleaf thistle |
Native
|
|
White or Elk thistle |
Native
|
|
Wyoming thistle |
Native
|
- *on Montana’s state noxious weed list.
- **on at least one county noxious weed list in Montana.
From a weed management perspective, differentiating among exotic thistles is important because a perennial rhizomatous thistle will require different control measures than a taprooted annual or biennial species. For example, taprooted species such as bull thistle may only live for a year or two, and simply cutting them with a hoe below the soil surface may kill them. In contrast, a patch of rhizomatous, perennial Canada thistle will require a long-term management plan to target its extensive root system.
Here are some key features used to identify thistles and a few tips to help determine if you have a native or exotic thistle:
- Bracts on flowering head. Bracts are tiny, leaf-like structures that that form a cup around the base of flowers. They are an important diagnostic feature of thistles. Often the size of individual bracts, height or width of bracts collectively, or structures that appear on bracts such as spines provide evidence if the thistle is exotic or native.
- Structures along the stem. Some exotic thistles have spiny wings that extend along the entire length of the stem. Native thistles may have short sections of the stem that appear winged, but wings will not extend along the entire length of the stem.
- Root system and growth habit. Canada thistle is an exotic rhizomatous thistle that is on the Montana noxious weed list. Rhizomatous species spread by underground shoots that develop some distance from the mother plant. No native thistles are rhizomatous, and they will generally not be found in extensive dense patches.
- Habitat characteristics. In general, native thistles grow in less disturbed areas than exotics do. However, some native thistles can also colonize disturbed areas like roadsides. Habitat alone is not a satisfactory way to tell native from exotic thistles, but it can be a clue.

Figure 5. Musk thistle (Carduus nutans) infestation along a road. Photo by Jane Mangold, Montana State University.
These tips are adapted from Guide to Exotic Thistles of Montana and How to Differentiate from Native Thistles (Parkinson and Mangold 2015, publication EB0221), a publication from Montana State University Extension that is designed to help identify exotic, invasive thistles and verify whether a thistle is native or exotic, thus helping to determine if control strategies are necessary. The publication includes a tutorial of how to use a simplified dichotomous key and a brief description and photos of the important anatomical features and terms that are needed to successfully identify the thistle. Photos of all 15 thistles found in Montana and brief descriptions of habitat are included. Printed copies of the thistle publication are free and can be ordered from Montana State University Extension Publications at (406) 994-3273, or on the MSU Extension Store.
Further Information
As always, if you have a question about thistle identification or need help with identification of any plant, contact your county or reservation Extension agent or the Schutter Diagnostic Lab for assistance.
Pest Management Tool Kit
Events and opportunities throughout Montana
Bighorn County Initial Private Applicator Training Program
June 16th. Harden, Montana. This program prepares and licenses private pesticide applicators to apply restricted use pesticide products on land they own, rent or lease. Contact Molly Hammond at (406) 665-9770 for more information.
2016 Pest Management Tour
The MSU Pesticide Education Program is offering multiple 6-credit program opportunities throughout eastern Montana in early October 2016. Region 4 private applicators must have 6 re-certification credits prior to the December 31, 2016, deadline to qualify into the next cycle. This may be the last chance for private applicators to accumulate credits prior to the December 31 deadline in eastern Montana. By August 2016, you may pre-register or see the online agenda. Contact your local Extension office for more information as October approaches.
Three New Weed-Related Publications from MSU Extension:
- Pipeline Reclamation (PDF) provides step-by-step instruction for managing risks associated with pipeline development, with a special focus on weed management and revegetation. Publication MT201602AG.
- Tall Buttercup: Identification, Biology, and Integrated Management (PDF) describes the biology and ecology of this exotic perennial forb and offers management recommendations. Publication MT201502AG.
- Watch out for Phragmites (PDF) provides information about the invasive grass Phragmites and gives tips to help identify native versus exotic Phragmites. Publication 4611.
All three publications (plus many more) are available at the Montana State University Extension store. Search by publication number or title.
MSU Extension Level 2 Noxious Weed Management Workshop
September 28-30, 2016, Comfort Inn, Bozeman, MT. Visit the MSU Extension Invasive Plants website for more information.
Northern Rockies Invasive Plant Council Conference
October 17-20, 2016, at the Boise Centre, Boise, ID. Visit the NRIPC website for more information.
Montana Realtor Noxious Weed Training Online Course
This 5-module course is specifically designed to provide realtors with fundamental knowledge about noxious weeds. It includes modules on noxious weed basics, plant and weed identification, the county noxious weed control act, and integrated weed management. Register online.
Ask the Expert
Questions from the community, answered by IPM specialists.
Question 1
I’ve heard the Environmental Protection Agency is requiring Montana private applicators to adhere to higher educational requirements. I heard this includes accumulating more credits per cycle, meeting categorical requirements and a shorter certification cycle. Is this true?
Cecil Tharp says: Yes and No. The EPA has proposed changing the minimum requirements for private applicators through a proposed change in federal rule (40 CFR 171). In Montana the proposal, if finalized, would move the private applicator certification cycle from a 5-year to a 3-year certification cycle, require 6 credits per cycle in core and 3 credits in each category certified (if the category is required), require applicators to accumulate half the credits in the last half of the cycle, and would require categorical training in certain subject areas (non-soil fumigation, soil fumigation, M-44, aerial and aquatic). There are many more changes proposed that can be reviewed on the EPA website at by searching "EPA proposed pesticide rule changes." Applicators should keep in mind that this is a proposal, not the final rule. EPA is reviewing comments prior to posting the final decision around October 2016. Stay tuned for an alert from MSU Extension Pesticide Education regarding EPA’s final decision.
Question 2
Last year I applied Varro to control weeds in my durum wheat. I had good results but in areas under drought conditions I noticed severe crop stress. Do you have any advice?
Fabian Menalled says: Varro (thiencarbazone-methyl) is a post emergence herbicide that provides control of certain grasses and broadleaf weeds including barnyardgrass, green foxtail, yellow foxtail, and wild oat and it can be used in winter wheat and spring wheat (including durum). One of its advantages is that it can be rotated to pulses in a relative short amount of time (9 months for lentils and peas). However, under drought conditions, late applications of Varro tank-mixed with emulsifiable concentrate (EC) formulations resulted in crop damage in durum wheat. Because of that, the new Varro label for Montana indicates that this product should be applied to all wheat from first leaf stage up to jointing stage. Also, you should avoid tank mix combinations with EC products on durum wheat if temperatures are below 32°F or above 85°F.
For more information about this product including rotation intervals, please consult the Varro label (2.1MB PDF).
Question 3
We have two Russian olive trees growing on our property. How do their seeds spread and what is the best way to get rid of these two trees? They are about 15 feet tall.
Jane Mangold says: Russian olive is a regulated plant on the Montana noxious weed list. This means it is unlawful to intentionally plant Russian olive, but a landowner is not mandated by law to control it. However, Russian olive has potentially significant negative impacts, especially in riparian areas, so getting rid of the trees on your property will probably make your neighbors and county weed coordinator very happy. Russian olive seeds are encased in the fruit or “olive” and are spread primarily by wildlife that eats the fruits. Seeds can also be dispersed by water. The best way to control your trees is the cut-stump method: cut the tree down as close to the ground as possible then coat the stump with herbicide. Concentrated glyphosate, triclopyr, or imazapyr is recommended. You must apply the herbicide within minutes of cutting, otherwise Russian olive will re-sprout. Consult the herbicide label for specific directions as sites and uses vary by product.
Question 4
Are seed treatments needed in wheat and peas?
Mary Burrows says: Seed treatment fungicides are very important for mitigation of seedborne and soilborne plant pathogens. In cereals, the smuts and bunts have been virtually eliminated since the introduction of systemic seed treatment fungicides. For pulse crops, it is important to use seed treatments for numerous seedborne fungi, especially those with long-lived survival structures such as Sclerotinia white mold, Botrytis grey mold, and Fusarium wilt. Seed testing and the use of seed treatment fungicides has helped us manage the threat of Ascochyta blight for many years. With increasing acres in pulse production and limited seed availability in 2016, many poor-quality seed lots are likely to be planted. Using a fungicide can help reduce the risk of disease and/or delay disease development.
There are seed treatment options for organic producers, but there is very little efficacy data available to make judgments on whether or not they will be useful. On chickpea in Montana, five biological controls available in the mid-2000s were tested, but none were effective against damping of in the field or greenhouse. Desi chickpeas are naturally more resistant to damping of than kabuli chickpeas.
Seed treatment fungicides protect seedlings against soilborne plant pathogens such as Pythium, Rhizoctonia, and Fusarium damping of and root rot for 2-3 weeks after planting, depending on the fungicide used. Efficacy data for cereals can be found in the Small Grain Seed Treatment Guide, MSU Extension Publications MontGuide MT199608AG (PDF). A summary table for pulse crop seed treatments can be found on the Montana AgAlert website.
Glyphosate-Resistant Conyza canadensis (marestail, horseweed) Confirmed in NE Montana
by Timothy Fine, Richland County Extension Agent, and Fabian Menalled, Cropland Weed Extension Specialist
Biotypes of Conyza canadensis (marestail, horseweed, or Canadian horseweed) with up to a five-fold increase in resistance to glyphosate, the active ingredient of Roundup® and other herbicides, have recently been confirmed in Richland County, Montana.
Marestail is an annual plant belonging to the Asteraceae family, and it is native to North America. As a winter or summer annual species, marestail emerges in fall or early spring, but it also can germinate in midsummer if growing conditions are adequate. In general, marestail plants start to bolt in April/May, begin to flower in July, set and disperse seed from August to October, and then die. Marestail plants can produce up to 200,000 seeds that are transported by wind, providing for effective spread of herbicide-resistant populations. Reports indicate that marestail seeds can easily travel more than 100 miles in a single fight with moderate wind speeds.
As a native to temperate regions, marestail plants can be found throughout southern Canada, the United States, and tropical America. In recent years, due to the spread of resistant biotypes, marestail has become a challenging weed to manage in reduced-till and non-till cropping systems. In Montana, marestail has primarily been reported in Richland, Valley, and Phillips Counties where it colonizes croplands, disturbed meadows, grasslands, and roadsides.
In the U.S. glyphosate resistance in marestail was first confirmed in 2000 in Delaware. Since then glyphosate-resistant marestail has been documented in more than one-third of the continental U.S. In all cases, the evolution of glyphosate resistance in marestail occurred in row crop systems, including cases of multiple herbicide resistance. This is the first confirmation of glyphosate-resistant marestail in Montana.
Herbicide resistance is defined by the Weed Science Society of America as “the inherited ability of a plant to survive and reproduce following exposure to a dose of herbicide normally lethal to the wild type. In a plant, resistance may be naturally occurring or induced by such techniques as genetic engineering or selection of variants produced by tissue culture or mutagenesis.” The full distribution of herbicide-resistant marestail biotypes in Montana and the mechanisms driving this resistance are still unknown. Whether glyphosate resistance was selected in Montana or it moved from other states, its selection occurred due to the over-reliance of glyphosate and the failure to develop effective integrated weed management programs.
MSU Extension offers the MontGuide, Preventing and Managing Herbicide-Resistant Weeds in Montana, which is available free at county and reservation Extension offices or the Extension Cropweeds website. The website weedscience.org, from the International Society of Weed Scientists, is also an excellent resource for more information on herbicide-resistant weeds.
If you have questions on preventing or managing herbicide-resistant weeds, please contact your local Extension office or Fabian Menalled at (406) 994-4783.
Meet Your Specialist
Jessica Rupp, Extension Potato, Sugar beet, and Pulse Crop Pathology

Where/when did you receive your degrees?
I began my studies in the field of biochemistry and cellular and molecular biology. I received a double Bachelor’s from Pittsburg State University in 2009. From there, I moved to Manhattan, Kansas to pursue a Ph.D. in Plant Pathology at Kansas State University.
What is your field of interest (scholastic or research)?
My responsibilities here at MSU encompass seed potato, sugar beet, and pulse crops. My work and research are split between two different avenues. First, I am committed to supplying growers with the information they need in the field. That means my lab is focused on applied field research emphasizing some of the greatest challenges my stakeholders encounter. Second, my lab is pursuing precision genome editing to create potato and sugar beet lines that lack genes that ultimately make them susceptible to a pathogen. We are just delving into this new area and we’re looking forward to seeing the system up and running in the lab.
When did you arrive in Bozeman?
I arrived in Bozeman in September 2015. It was an exciting time to arrive. My first day of work was spent on the beet harvester in Huntley with my predecessor, Dr. Barry Jacobsen. Around that time, potato growers were harvesting as well. Bozeman is wonderful, and there are many times I just look around to remind myself of where I live!
What are some important areas of focus in your field?
Many important diseases affect potato and sugar beet. My current focus in potato centers on Potato virus Y (PVY). PVY is the type member of the potyvirus family. Potyviruses are fascinating because they have so few genes, and yet we cannot solve this problem! Additionally, we need to be ever mindful of Late Blight of potato. Montana is a seed producing state, so it is imperative we are taking all the IPM steps to ensure our top quality potatoes in the market. Sugar beet has some struggles as well. Between foliar problems, like Cercospora leaf spot, and root problems, like Rhizoctonia root and crown rot, there is a lot to tackle.
What projects would you like to focus on in the future?
I would like to work on projects that anticipate the future as much as I am able. I plan to look at disease complexes and not just single diseases. Pathogens are out there fighting to get established, so they are not just fighting the plant and the environment, but each other, too! I plan to continue applied field research and precision genome editing. I have more ideas up my sleeve, but folks will just have to wait and see how those pan out in the future.
Where are you from originally?
I am originally from southeast Kansas. Those of you that have met me, know I have an accent! I was born and raised in a small college town called Pittsburg. Today Pittsburg is famous for the Pittsburg State University Gorillas, the most winning football team in the state, and fried chicken. My dad was a professor, and I was raised a Gorilla. I didn’t plan on moving to another college town named after a different town, but I ended in Manhattan, KS, “The Little Apple.” A fun fact is that I lived on Montana Street in Kansas!
What are some of your current projects?
I am continuing studies that Dr. Jacobsen began with Dr. Ken Kephart using seed treatments for sugar beet. Additionally, Dr. Kephart and I will be investigating the role that application method plays in disease control. I am also planning on testing some seed treatments for Rhizoctonia on potato. In the lab, we are using techniques of precision genome editing of potato and sugar beet to try and get resistance. A key feature of our method is that it isn’t considered transgenic. With the current climate of GMO being what it is, I think that we should be pursuing every avenue we can to help growers.
How can farmers use your research to their benefit?
Timing is a key element for farmers. They juggle so many things, I hope my research can provide them with more peace of mind. I want to fine tune optimal timing conditions with as many tools as growers have available. If I can give them more time to get an application on the field I will have done something great. I also hope to provide them with materials that make planning and decision making easier.
Acknowledgments
Do You Have a Question or Comment Regarding the Montana IPM Bulletin?
Send inquiries and suggestions to:
Cecil Tharp
Pesticide Education Specialist
P.O. Box 172900
Montana State University
Bozeman, MT 59717-00
Phone: (406) 994-5067
Fax: (406) 994-5589
Email: [email protected]
Web: pesticides.montana.edu
Jane Mangold
Invasive Plant Specialist
P.O. Box 173120
Montana State University
Bozeman, MT 59717-3120
Phone: (406) 994-5513
Fax: (406) 994-3933
Email: [email protected]
Web: landresources.montana.edu
Noelle Orloff
Associate Extension Specialist
P.O. Box 173120
Montana State University
Bozeman, MT 59717-3120
Phone: (406) 994-6297
Fax: (406) 994-3933
Email: [email protected]
Web: diagnostics.montana.edu

Common chemical and trade names are used in this publication for clarity by the reader. Inclusion of a common chemical or trade name does not imply endorsement of that particular product or brand of herbicide. Recommendations are not meant to replace those provided in the label. Consult the label prior to any application.
Original Spring 2016 PDF (2.8MB)
